Organs, Tissues and Cells (apart from embryos and gametes) framework
This briefing discusses the Organs, Tissues and Cells (apart from embryos and gametes) framework. The Organs, Tissues and Cells framework relates to policy on the safety and quality of organs, tissues and cells (excluding reproductive tissues and cells). The framework also sets out arrangements for co-operation between officials in the UK Government and devolved administrations. The briefing also provides background information on the common frameworks programme.
Summary
This briefing provides detailed information on the Organs, Tissues and Cells (apart from embryos and gametes) framework. The Health, Social Care and Sport Committee led on scrutiny of this framework.1
Background information on, for example, what common frameworks are and how they have been developed is also provided in this paper. The policy context of the framework is also briefly covered in this briefing.
The SPICe common frameworks hub collates all publicly available information on frameworks considered by committees of the Scottish Parliament.
In session five the Finance and Constitution Committee reported on common frameworks and recommended that frameworks should include the following:
their scope and the reasons for the framework approach (legislative or non-legislativei) and the extent of policy divergence provided for;
decision making processes and the potential use of third parties;
mechanisms for monitoring, reviewing and amending frameworks including an opportunity for Parliamentary scrutiny and agreement;
the roles and responsibilities of each administration; and
the detail of future governance structures, including arrangements for resolving disputes and information sharing
The Scottish Government’s response highlighted that there may be a "range of forms" which frameworks could take.
More detail on the background to frameworks is available in a SPICe briefing and also in a series of blogs available on SPICe spotlight.
The Organs, Tissues and Cells Common Framework covers policy previously covered by EU legislation designed to set minimum standards for the safety and quality of organs, tissues and cells. The framework sets out how the four governments propose to work together on organs, tissues and cells policy so that organs, tissues and cells can continue to be shared across the UK.
The framework is a non-legislative agreement formalising ways of working between the four governments on organs, tissues and cells policy. The framework sets out the scope of the policy area, the roles and responsibilities of parties to the framework, as well as the mechanisms for monitoring, review, amendment, and dispute resolution.
What are common frameworks?
A common framework is an agreed approach to a particular policy, including the implementation and governance of it. The aim of common frameworks is to manage divergence in order to achieve some degree of consistency in policy and practice across UK nations in areas formerly governed by EU law.
In its October 2017 communique on common frameworks, the Joint Ministerial Committee (EU Negotiations) (JMC (EN)) stated that:
A framework will set out a common UK, or GB, approach and how it will be operated and governed. This may consist of common goals, minimum or maximum standards, harmonisation, limits on action, or mutual recognition, depending on the policy area and the objectives being pursued. Frameworks may be implemented by legislation, by executive action, by memorandums of understanding, or by other means depending on the context in which the framework is intended to operate.
Joint Ministerial Council (EU Negotiations), 16 October 2017, Common Frameworks: Definition and Principles
The Scottish Government indicated in 2019 that common frameworks would set out:
the area of EU law under consideration, the current arrangements and any elements from the policy that will not be considered. It will also record any relevant legal or technical definitions.
a breakdown of the policy area into its component parts, explain where the common rules will and will not be required, and the rationale for that approach. It will also set out any areas of disagreement.
how the framework will operate in practice: how decisions will be made; the planned roles and responsibilities for each administration, or third party; how implementation will be monitored, and if appropriate enforced; arrangements for reviewing and amending the framework; and dispute resolution arrangements.
However, the Food and Feed Safety and Hygiene Law framework outline considered by the session five Health and Sport Committee noted that:
the framework itself is high level and commits all signatories to early, robust engagement on policy changes within scope.
Framework Outline Agreement and Concordat, 30 November 2020
The framework outline went on to note that the framework:
is intended to facilitate multilateral policy development and set out proposed high level commitments for the four UK Administrations. It should be viewed as a tool that helps policy development, rather than a rigid template to be followed.
As such, it is likely that there will be significant variation between frameworks in terms of whether they set policy or set out how decisions on policy within the scope of the framework will be taken.
There are, however, similarities between frameworks in terms of their overall structure, with the agreements setting out the roles and responsibilities for parties to the framework, how the framework can be reviewed and amended, and how disputes are to be resolved.
Why are common frameworks needed?
During its membership of the European Union, the UK was required to comply with EU law. This means that, in many policy areas, a consistent approach was often adopted across all four nations of the UK, even where those policy areas were devolved.
On 31 December 2020, the transition period ended, and the United Kingdom left the EU single market and customs union. At this point, the requirement to comply with EU law also came to an end. As a result, the UK and devolved governments agreed that common frameworks would be needed to avoid significant policy divergence between the nations of the UK, where that would be undesirable.
The Joint Ministerial Committee (JMC) was a set of committees that comprised ministers from the UK and devolved governments. The JMC (EU Negotiations) sub-committee was created specifically as a forum to involve the devolved administrations in discussion about the UK’s approach to EU Exit. Ministers responsible for Brexit preparations in the UK and devolved governments attended these meetings.
In October 2017, the JMC (EN) agreed an underlying set of principles to guide work in creating common frameworks. These principles are set out below.
Common frameworks will be established where they are necessary in order to:
enable the functioning of the UK internal market, while acknowledging policy divergence;
ensure compliance with international obligations;
ensure the UK can negotiate, enter into and implement new trade agreements and international treaties;
enable the management of common resources;
administer and provide access to justice in cases with a cross-border element; and
safeguard the security of the UK.
Frameworks will respect the devolution settlements and the democratic accountability of the devolved legislatures, and will therefore:
be based on established conventions and practices, including that the competence of the devolved institutions will not normally be adjusted without their consent;
maintain, as a minimum, equivalent flexibility for tailoring policies to the specific needs of each territory, as is afforded by current EU rules; and
lead to a significant increase in decision-making powers for the devolved administrations.
What is the process for developing frameworks ?
Frameworks are inter-governmental agreements between the UK Government and the devolved administrations.
They are approved by Ministers on behalf of each government prior to being sent to all UK legislatures for scrutiny.
The UK Government Cabinet Office is coordinating the work on developing common frameworks.
Common frameworks go through four phases of development before implementation at phase five. The stages are set out below. The parliament receives frameworks for scrutiny at phase four.
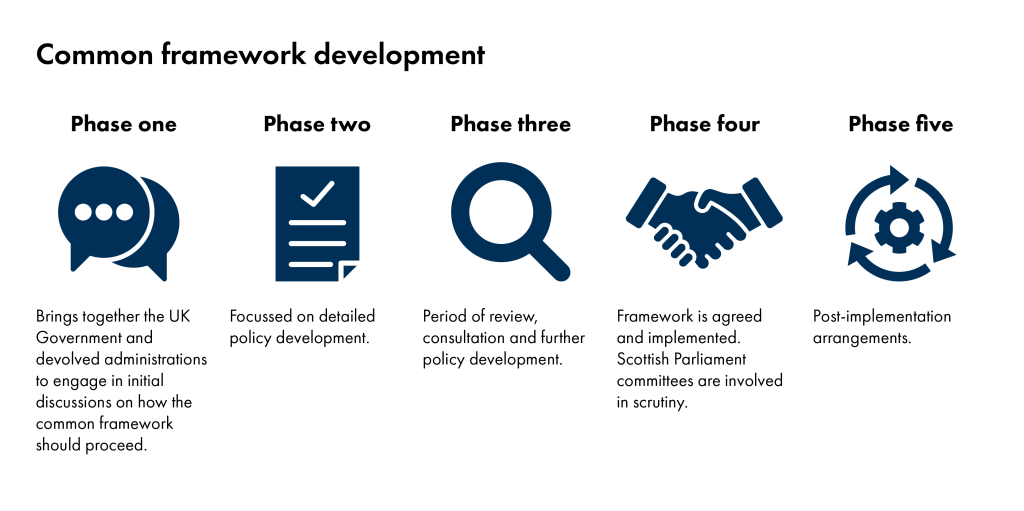
How will the Scottish Parliament consider frameworks?
Frameworks which have reached phase four are available to be considered by the Scottish Parliament. Subject committees can consider frameworks which sit within their policy areas.
Each legislature in the UK can consider common frameworks. Issues raised by legislatures during this scrutiny are fed back to their respective government. Governments then consider any changes which should be made to frameworks in light of scrutiny by legislatures before implementing the framework. Changes in light of scrutiny are not, however, a requirement.
The Constitution, Europe, External Affairs and Culture Committee has an oversight role in relation to frameworks and will lead on cross-cutting issues around transparency, governance and ongoing scrutiny.
The Scottish Government has previously acknowledged the ongoing role of the Scottish Parliament in relation to frameworks:
Consideration will also need to be given to what role the Parliament might have in the ongoing monitoring and scrutiny of frameworks post-implementation.
Scottish Government response to the session five Finance and Constitution Committee report on common frameworks, June 2019
The Scrutiny Challenge
The way in which common frameworks have been developed and will operate raises some significant scrutiny challenges for the Scottish Parliament.
Common frameworks are intergovernmental agreements and the scope for parliamentary influence in their development is significantly limited with scrutiny taking place at phase four.
The ongoing operation of frameworks will take place at an official level between government departments. It is therefore unclear how much information the Parliament may be able to access to scrutinise the effect of frameworks on policy-making.
The Scottish Government and the UK Government have differing objectives in relation to frameworks. The UK Government is seeking “high levels of regulatory coherence”.1 The Scottish Government believes that they are about “allowing legitimate policy choices”.1
The interconnected nature of common frameworks and the UK Internal Market Act 2020 (see section on the UK Internal Market Act).
The impact of common frameworks on the Scottish Government’s stated policy position of keeping pace with EU law.
The fact that most frameworks have been operating on an interim basis since 1 January 2021 in spite of being unavailable for scrutiny by legislatures3.
The legacy expert panel report to the session five Finance and Constitution Committee noted these scrutiny challenges. The Committee had previously recommended that the Scottish Government should have to report on the operation of each common framework, noting interactions with cross-cutting issues such as keeping pace with EU law, on an annual basis.
Scrutiny at other legislatures
The Organs, Tissues and Cells Framework (the focus of this briefing) was considered by the House of Lords Common Frameworks Scrutiny Committee in May 2021. The views of the committee are discussed in the Framework Analysis section.
The UK Internal Market Act 2020
The UK Internal Market Act 2020 was introduced in the UK Parliament by the UK Government in preparation for the UK’s exit from the EU. The Act establishes two market access principles to protect the flow of goods and services in the UK’s internal market.
The principle of mutual recognition, which means that goods and services which can be sold lawfully in one nation of the UK can be sold in any other nation of the UK.
The principle of non-discrimination, which means authorities across the UK cannot discriminate against goods and service providers from another part of the UK.
The Act means that the market access principles apply even where divergence may have been agreed in a framework.
The introduction of the UK Internal Market Act had a significant impact on the common frameworks programme because of the tension between the market access principles contained in the Act and the political agreement reached that “common frameworks would be developed in respect of a range of factors, including "ensuring the functioning of the UK internal market, while acknowledging policy divergence".i
UK Government Ministers have the power to disapply the market access principles set out in the Act where the UK Government has agreed with one or more of the devolved governments that divergence is acceptable through the common frameworks process.
Although UK Ministers can disapply the market access principles in such circumstances, they are not legally obliged to do so.
On 2 December 2021, Angus Robertson MSP, Cabinet Secretary for Constitution, External Affairs and Culture wrote to the Convener of the Constitution, Europe, External Affairs and Culture Committee to give an update on the common frameworks programme.
The letter indicated that at a recent Ministerial quadrilateral, agreement had been reached between the UK Government and the Scottish Government and other devolved administrationsii on an approach to "securing exemptions to the Act for policy divergence agreed through common frameworks".
The meeting agreed an approach to securing exemptions to the Act for policy divergence agreed through common frameworks, and endorsed the text of a statement that UK Ministers will shortly make to the House of Commons. This will give effect to firm commitments made to the UK Parliament during the passage of the Bill that “…divergence may occur where there is agreement under a common framework, and that such divergence could be excluded from the market access principles. Regulations to give effect to such an agreement can be made under Clauses 10 and 17. In those cases, the Secretary of State would be able to bring to the House a statutory instrument to exclude from the market access principles a specific agreed area of divergence.
This would follow consensus being reached between the UK Government and all the relevant parties that this is appropriate in respect of any specific defined topic within a common framework.
Letter from the Cabinet Secretary for Constitution, External Affairs and Culture, 2 December 2021
Process for considering UK Internal Market Act exclusions in common framework areas
The UK Government and devolved administrations have agreed a process for considering exclusions to the market access principles of the UK Internal Market Act 2020. The process was published on 10 December 2021.
The process requires that if a party to the framework wishes to seek an exclusion to the market access principles, it must set out the scope and rationale for this. The proposed exclusion is then considered by the appropriate framework forum, taking into account evidence including about the likely direct and indirect economic impact of the proposed exemption. If the exemption is agreed, it is for UK Ministers to introduce a draft instrument to the UK Parliament to give effect to the exclusion. The UK Parliament will then consider the draft instrument.
The process is set out in full below.
Proposal and consideration of exclusions
1. Sections 10 and 18 and Schedules 1 and 2 of the UK Internal Market Act contain provisions excluding the application of the United Kingdom market access principles in certain cases.
2. Whenever any party is proposing an amendment to those Schedules in areas covered by a Common Framework:
a. the exclusion seeking party should set out the scope and rationale for the proposed exclusion; and
b. consideration of the proposal, associated evidence and potential impact should be taken forward consistent with the established processes as set out in the relevant Common Framework, including an assessment of direct and indirect economic impacts.
3. It is recognised that all parties will have their own processes for considering policy proposals. Administrations should consult and seek agreement internally on their position before seeking to formally agree the position within the relevant Common Frameworks forum.
Agreement of an exclusion request
4. Where policy divergence has been agreed through a Common Framework this should be confirmed in the relevant Common Framework forum. This includes any agreement to create or amend an exclusion to the UKIM Act 2020’s market access principles.
5. Evidence of the final position of each party regarding any exclusion and whether an agreement has been reached should be recorded in all cases. This could take the form of an exchange of letters between appropriate UK Government and Devolved Administration ministers and include confirmation of the mandated consent period for Devolved Administration ministers regarding changes to exclusions within the Act.
6. Parties remain able to engage the dispute resolution mechanism within the appropriate Common Framework if desired.
Finalising an exclusion
7. Under section 10 or section 18 of the UK Internal Market Act 2020 amendments to the schedules containing exclusions from the application of the market access principles require the approval of both Houses of the UK Parliament through the affirmative resolution procedure. Where agreement to such an exclusion is reached within a Common Framework, the Secretary of State for the UK Government department named in the Framework is responsible for ensuring that a draft statutory instrument is put before the UK Parliament.
Uk Government . (2021, December 10). Guidance: Process for considering UK Internal Market Act exclusions in Common Framework areas. Retrieved from https://www.gov.uk/government/publications/process-for-considering-ukim-act-exclusions-in-common-framework-areas/process-for-considering-uk-internal-market-act-exclusions-in-common-framework-areas#agreement-of-an-exclusion-request [accessed 13 December 2021]
Organs, Tissues and Cells (apart from embryos and gametes)
The Organs, Tissues and Cells (apart from embryos and gametes) framework ("the framework") has reached phase four and has, as such, been received by the Scottish Parliament for scrutiny. Scrutiny was undertaken by the Health, Social Care and Sport Committee.
The framework has also been received by other UK legislatures.
This briefing is intended to facilitate scrutiny of the framework by the Scottish Parliament.
Policy Area
The framework concerns policy on the safety and quality of organs, tissues and cells, excluding reproductive tissues and cells. It seeks to maintain a compatible minimum set of safety and quality standards between the UK Government and devolved governments to make it easier for organs, tissues and cells to continue to be shared across the UK.
Minimum standards ensure a high level of health protection. The UK Government, Scottish Government, Welsh Government and Northern Ireland Department of Health are obliged to ensure that quality and safety standards are maintained in accordance with the retained EU law (see section on Scope). To facilitate this, the framework sets out arrangements for co-operation between officials in the UK Government and devolved governments.
The framework is intended to allow for necessary divergence by one or more governments to address public health concerns that only affect certain parts of the UK. As such, it sets out a process for one or more governments to suggest changes to standards and how such a proposal will be considered.
Scope
The framework encompasses and relates to two EU Directives on the minimum safety and quality standards for organs, tissues and cells for treating patients.i
EU Tissues and Cells Directive (2004/23/EC) ("the European Tissues and Cells Directive"): covers issues such as donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells intended for human application.
EU Organs Directive (2010/53/EU) ("the European Organs Directive"): covers all steps in the organ transplantation process from donation, procurement, testing, characterisation, preservation, transport and finally transplantation.
The two main pieces of EU legislation that formerly governed this policy area and intersect with devolved competence are:
Directive 2004/23/EC (‘the European Tissues and Cells Directive’)
Directive 2010/53/EU (‘the European Organ Directive’).
The associated implementing directives also intersect with devolved competence and detail the scope of this policy area:
Tissues and cells
Commission Directive 2006/17/EC: regarding certain technical requirements for the donation, procurement and testing of human tissues and cells.
Commission Directive 2006/86/EC: concerning traceability requirements, notification of serious adverse reactions and events, additional technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells.
Commission Directive 2012/39/EU: amending Directive 2006/17/EC as regards certain technical requirements for the testing of human tissues and cells.
Commission Directive 2015/565: amending Directive 2006/86/EC as regards certain technical requirements for the coding of human tissues and cells.
Commission Directive 2015/566: implementing Directive 2004/23/EC concerns the procedures for verifying the equivalent standards of quality and safety of imported tissues and cells.
Commission Decision 2010/453/EC: establishing guidelines concerning the conditions of inspections and control measures, and on the training and qualification of officials, in the field of human tissues and cells.
Commission Decision (2015) 4460: establishing a model for agreements between the Commission and relevant organisations on the provision of product codes for use in the Single European Code (SEC).
The SEC is a unique identifier that consists of a donation identification sequence (indicating the origin of the tissue or cells) and a product identification sequence (classifying the type of tissue or cells).
Organs
Commission Directive 2012/25/EU: regarding the information procedures for exchange of human organs intended for transplantation, between EU countries.
Enforcement was delegated to the UK-wide regulator, the Human Tissue Authority (HTA). The HTA remains the competent authority for monitoring compliance with retained EU law relating to the safety and quality of organs, tissues and cells. With the requirement to comply with EU law now at an end, the UK and devolved governments have wider scope to make changes to the safety and quality regulation of organs, tissues and cells.
The framework notes the following interdependencies:
The Blood Safety and Quality Provisional Common Framework: there are joint UK-wide groups that advise Ministers and health departments on the most appropriate ways to ensure the safety of blood, cells, tissues and organs for transfusion and transplantation.
Medical devices legislation: reagents (medical devices) are used in the collection and processing of organs, tissues and cells.
Definitions
The framework defines the following key terms:
Concordat: Joint non-legislative agreement that gives effect to the Common Framework.
Summary of proposed approach
The framework comprises both legislative and non-legislative mechanisms.
The framework identifies that this policy area requires a common framework to:
"enable the functioning of the UK internal market, while acknowledging policy divergence: for organs, tissues and cells this will enable the transplant material to be shared around the UK;
...safeguard the security of the UK: for organs, tissues and cells, the sharing of serious adverse events or reactions (SAERs) information to maintain patient safety."
The underlying principle of the proposed approach is of collaborative working between the four governments. Part of this collaborative working principle is the agreement not to introduce changes to safety and quality standards legislation without first discussing proposals with the other parties to the framework.
The framework therefore describes governance structures and process for discussing and managing the impact of any proposed policy changes by one or more governments for the whole of the UK. As the framework is intended to ensure a set of minimum standards for the safety and quality of organs, tissues and cells (so that organs, tissues and cells can be shared across the UK) the joint decision making and dispute avoidance mechanisms include points at which scientific and risk assessment advice can be solicited in the event changes to policy are suggested.
The same governance structures are expected to apply when regulations change in Northern Ireland as a result of EU alignment.
Northern Ireland will remain aligned with EU regulations on safety and quality of organs and tissues and cells in respect of The Protocol on Ireland/Northern Ireland. In supporting material provided to the Health, Social Care and Sport Committee on 9 December 2021 by the Minister for Public Health, Sport and Wellbeing Maree Todd MSP, parliamentary committees were encouraged to note:
No issues are particularly contentious, although the impact of the Northern Ireland Protocol (NIP) on the ability of the Northern Ireland Executive to make changes to Regulations in Northern Ireland will depend on the nature of any future proposals for legislative change (either from other UK nations or the EU). The NIP specifies that Northern Ireland must follow EU rules on the safety and quality of blood, organs, tissues and cells. As changes are made to areas in scope of those rules, they will apply directly, or require implementation, in Northern Ireland as they do now.
The House of Lords Common Frameworks Scrutiny Committee raised its concerns on matters concerning the Northern Ireland Protocol and the lack of engagement with stakeholders in Northern Ireland and the Republic of Ireland in correspondence from 20 January 2022 to Neil O'Brien MP (the Parliamentary Under Secretary of State Department for Levelling Up, Housing and Communities):
Likewise, the provisional frameworks on ‘Organs, Tissues and Cells’ and ‘Blood Safety and Quality Provision’ do not set out “the importance of cross-border healthcare delivery in, for example, work of All-Island Congenital Heart Disease Network or North-South Living Donor Exchange Kidney Transplant Service both of which meet under the auspices of Strand Two of 1998 Agreement and whose work is relevant for the operation of these CFs”.
Stakeholder engagement
The framework's supporting material sent to the Health, Social Care and Sport Committee on 9 December 2021 describes the stakeholder engagement that took place during phase 3 of the development of the framework. Stakeholder consultation was coordinated by the UK Government Department of Health and Social Care on behalf of the four governments. Feedback on the framework was gathered in November 2020. The stakeholders engaged in the framework's phase 3 feedback process included:
NHS Blood and Transplant
the Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO)
the Joint United Kingdom Blood Transfusion and Tissue Transplantation Services Processional Advisory Committee (JPAC)
Scottish National Blood Transfusion Service (SNBTS)
Human Tissue Authority (HTA)
Welsh Transplantation Advisory Group
Cardiff and Vale University Health Board
Health and Social Care Northern Ireland (HSCNI)
the Welsh Renal Clinical Network
The supporting material also notes the evidence session held by the House of Lords Common Frameworks Scrutiny Committee on 25 May 2021 (see Scrutiny at other legislatures) and indicates that future stakeholder engagement will take place once scrutiny of the framework by UK legislatures is complete.
It is not clear from the framework documents what stakeholder engagement will be necessary for the ongoing functioning of the framework. However, the framework indicates:
There is a need for continued robust policy development encompassing policy and technical expertise from all four governments, including the need to fully assess the potential impacts of legislative changes on all affected stakeholders. Governments may wish to do this work individually or in collaboration before initiating a UK-wide discussion of a potential change to the standards.
The framework also notes that some stakeholder engagement should take place if one or more governments initiate a risk assessment and management process as part of decisions on policy divergence:
One or more governments may initiate a risk assessment process that should include discussions with the national transplant services and the regulator, as appropriate. The assessment should include seeking advice from the relevant scientific advisory bodies. Final decisions at the end of the risk assessment process should require collective sign-off (e.g. legislative or operational changes) by all Ministers across the UK. While the ability to diverge is always available to any individual government, it will be important for any diverging government to consider the impact on patient safety and confidence and the JMC(EN) Common Frameworks principles.
Detailed overview of proposed framework: legislation
This section provides information on the legislation associated with the framework.
The framework documents state that "no legislation to support the Framework is considered necessary". However, as previously noted, the framework agreement covers the subject matter of the EU Organs Directive (2010/53/EU) and EU Tissues and Cells Directive (2004/23/EC) and implementing acts. As such, it is understood that no additional legislation is required for the framework.
The Quality and Safety of Organs Intended for Transplantation (Amendment) (EU Exit) Regulations 2019/483 and The Human Tissue (Quality and Safety for Human Application) (Amendment) (EU Exit) Regulations 2019/481 ("the 2019 Statutory Instruments") retained safety and quality standards for organs and non-reproductive tissues and cells and ensured the regulatory framework could operate as intended following the UK's departure from the EU. The 2019 Statutory Instruments also transferred powers to update the quality and safety regulations to either the Secretary of State for Health and Social Care on behalf of the UK or to Ministers in each of the devolved governments.
The regulations have since been amended by The Quality and Safety of Organs Intended for Transplantation (Amendment) (EU Exit) Regulations 2020/1305 and The Human Tissue (Quality and Safety for Human Application) (Amendment) (EU Exit) Regulations 2020/1306 ("the 2020 Statutory Instruments") in respect of the Northern Ireland Protocol. The 2020 Statutory Instruments limit the powers granted to Ministers in the 2019 Statutory Instruments to authorities in Great Britain (i.e. UK Government, Welsh Government and Scottish Government). Northern Ireland is required to meet the requirements set out by the European Tissues and Cells Directive and the European Organ Directive and the implementing acts for as long as the Northern Ireland Protocol is in force.
Detailed overview of proposed framework: non-legislative arrangements
The UK Government, Scottish Government, Welsh Government and Northern Ireland Department of Health have agreed a Concordat to give effect to the framework. The Concordat sets out:
the intended ways of working within the framework;
the continuation of good working relations and open communication;
the maintenance of a compatible minimum set of high standards of safety and quality for organs and non-reproductive tissues cells;
a dispute avoidance and resolution mechanism;
and a review and amendment mechanism.
Organs, Tissues and Cells in practice
Decision-making
Decisions, including changes to legislation and policy, on the safety and quality standards for organs, tissues and cells within the policy area, can be made at a Ministerial level by individual governments. This includes, but is not limited to, the following:
For non-reproductive tissues and cells
updating technical requirements relating to tissues and cells;
prescribing traceability requirements and notification requirements in relation to SAERs; and
verifying equivalent standards of safety and quality where tissues and cells are imported from third countries.
For organs
updating requirements for organ and donor characterisation to mitigate risk to human health, usually in response to an emerging disease outbreak; and
responding to SAERs which present a serious risk to human health.
The framework sets out a process for when a government wants to make a change to the organs, tissues and cells safety and quality legislation. This process asks that the relevant government(s) wanting to make a change to legislation:
notify all governments, setting out details of the proposal and invite comments;
arrange a meeting with policy officials to discuss the detail of the proposals if a government requests this;
seek to agree a way forward on the issue; and
depending on the issue, seek input from the following:
advice from an advisory body, the regulator, the donation or blood services; and
consultation with stakeholders.
Officials, in line with the collaborative working principles set out by the framework and Concordat, are expected to share the advice so that each government can advise on the policy proposals and seek Ministerial decisions. The framework indicates that officials of all four governments are expected to submit the same common recommendations to Ministers for a decision (either for a common approach across the UK or divergent approaches).
The framework notes that if official level issues are not resolved in the joint decision-making process, then it can be referred to senior officials for further consideration. The issues identified in the framework include officials being unable to agree to assess a proposal, develop an approach for implementing a proposal, or disagreement over factual information in the advice being presented to Minsters. Following agreement at the official level (or senior official level), the recommendations can then be presented to Ministers for review. The framework indicates that policy officials can begin implementing the recommended proposal following agreement from Ministers.
The framework decision-making process indicates that if agreement cannot be reached at the official or senior official level, the issue can be escalated for discussion at the Ministerial level through the Dispute Resolution Process.
Roles and Responsibilities: parties to the framework
This section sets out the roles and responsibilities of each party to the framework.
The parties to the framework include the Officials, Senior Officials and Ministers (including Senior Ministersi where there is a distinction) from each of the four governments.
The framework notes that information sharing between parties to the framework is expected. Specifically, it is expected that each government will aim to provide each other with full and open access to scientific, technical and policy information (including statistics and research), and where appropriate, representations from third parties. The relevant advisory bodies for this framework are:
Officials
Official level meetings with policy teams across the four nations are expected to take place around SaBTO meetings. SaBTO normally meets three times a year. The framework notes official level meetings are intended to:
provide an opportunity to discuss organs, tissues and cells policy
discuss issues related to the framework
facilitate information sharing
share updates and consider the impact of any developments
consider prospective policy changes
assess emerging issues and share intelligence.
It is expected that officials will continue discussion on the policy area in the course of everyday business and hold specific ad hoc meetings whenever appropriate.
If and when a policy proposal arises, officials are expected to present advice to Ministers on the rationale for the approach taken (e.g. a UK or GB-wide approach) or why divergent policies may be necessary.
Senior Officials
The role of senior officials set out in the framework is to provide strategic direction on the policy area.
Senior official meetings will be convened on a regular or ad hoc basis.
Senior officials may also convene to discuss issues or disputes raised at the official level. Senior officials can also escalate issues to Ministers as part of the dispute resolution mechanism if necessary.
Ministers
Ministers may receive advice and approve proposals from their officials either concurrently across governments as issues arise, or in the course of business as usual work for individual governments.
Ministers also have a role in the joint decision-making process and dispute resolution process to review and discuss issues that are escalated to ministerial level.
Roles and responsibilities: existing or new bodies
This section sets out the roles and responsibilities of any bodies associated with the framework which already exist, or which are to be created.
In this framework, the bodies listed below are advisory and are independent of government. It is anticipated that they will provide scientific and expert advice to officials (through official level meetings), senior officials and Ministers.
Advisory Committee on the Safety of Blood, Tissues and Organs
The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO) provides advice to Ministers in the four governments of the UK on the most appropriate ways to ensure the safety of blood, cells, tissues and organs for transfusion and transplantation.
Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee
The purpose of the Joint United Kingdom Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee (JPAC) is:
To ensure that all relevant aspects of dealing with the safety of blood and tissues in the UK are covered, and that the professional advice from JPAC is communicated appropriately and in a timely fashion.
To prepare detailed service guidelines for the United Kingdom Blood Transfusion Services, taking account of the Blood Safety and Quality Regulations (2005), the Human Tissue (Quality and Safety for Human Application) Regulations 2007 and future UK legislation affecting the blood and tissue services.
To be an Advisory Committee to the United Kingdom Blood Transfusion Services, normally by reporting to the Medical Directors of the individual Services who are themselves individually accountable to the Chief Executives and Directors of the Services.
Monitoring and enforcement
The framework indicates that ad hoc official level meetings will take place to monitor the framework. The purpose of monitoring is to assess:
intergovernmental co-operation and collaboration as a result of the framework;
whether parties are implementing and complying with the framework;
whether divergence has taken place in contravention of the common framework principles;
whether divergence has taken place in contravention of the appropriate intergovernmental structures; and
whether harmful divergence has taken place that impacts on the policy area covered by the framework.
The outcome of this monitoring is expected to inform joint decision-making and the review and amendment process. The monitoring arrangements are also expected to identify when there is an unresolved disagreement for the dispute avoidance and resolution mechanism to address.
Review and amendment
This section sets out the Review and Amendment (RAM) process described in the framework.
The framework stipulates that an initial review should take place one year after the framework comes into effect to determine if the arrangements are functional. Following the initial review, there are two types of review that can take place. The process for agreeing amendments should be identical regardless of the type of review.
At the outset of the review stage, the Parties must agree timelines for the process, including the possible amendment stage. If a decision is not reached in either the review or amendment stage, parties to the framework can raise it as a dispute through the framework dispute avoidance and resolution process.
The framework and associated concordat states that:
The Review and Amendment Mechanism (RAM) ensures the Framework can adapt to changing policy and governance environments in the future.
Review Stage
Periodic Review: a periodic review of the framework will take place every two years and will be, in line with official or, if required, ministerial-level meetings.
The period of two years starts from the conclusion of a periodic review and any amendment stages that follow.
During the periodic review, parties to the framework should discuss whether the governance and operational aspects of the framework are working effectively, and whether decisions made over the previous two years need to be reflected in an updated non-legislative agreement.
Exceptional Review: an exceptional review of the framework is triggered by a significant issue.
A significant issue must be time sensitive and fundamentally impact the operation or the scope of the framework.
This kind of review may include a review of governance structures if all parties agree it is required. Otherwise, these issues are to be handled in the periodic review.
The same significant issue cannot be discussed within six months of the closing of that issue.
The amendment stage can only be triggered through unanimous agreement by Ministers. If parties agree that no amendment is required, the relevant time period begins again for both review types (i.e. it will be two years until the next periodic review and at least 6 months until the same significant issue can trigger an exceptional review).
The RAM relies on consensus at each stage of the process from the Ministers responsible for the policy areas covered by the framework. Third parties can be used to provide advice to the Parties at any stage in the process. These include other government departments or bodies as well as external stakeholders such as NGOs and interest groups.
Amendment Stage
Following the parties jointly deciding to enter the amendment stage, they will enter into discussion around the exact nature of the amendment. This can either be led by one party to the framework or all.
If an amendment is deemed necessary during either type of review (periodic or exceptional), the existing framework will remain in place until a final amendment has been agreed.
All amendments to the framework must be agreed by all parties and a new framework signed by all parties. If agreement cannot be reached on whether or how a framework should be amended this may become a disagreement and, as such, could be raised through the framework dispute avoidance and resolution mechanism.
Dispute resolution official level
This section considers the dispute resolution process set out within the framework.
There is a three-level process for resolving disagreements that is similar to the Decision-Making arrangements and follows the Roles and Responsibilities of Parties outlined in the framework. As such, there is an Official level, Senior Official level and Ministerial level for resolving disagreements before the matter would then be escalated to intergovernmental structures in the event of no agreement.
In line with the Concordat, it is expected that the four governments will aim to resolve differences and reach agreement at the lowest possible level (official level) through discussions. When an issue is raised at official level, consideration will be given to how quickly a decision is required. This assessment will guide timescales for escalation of disagreement within the governance structure, with decisions requiring a more immediate resolution (e.g. an emergency response) being escalated more quickly.
At each stage, further evidence may be requested from the preceding forum before the disagreement is discussed. JPAC and SaBTO may be used as third parties to provide scientific or technical advice to the parties to the framework. The Human Tissue Authority is also included in the framework as one of the third parties that can be consulted for scientific and technical advice during the Dispute Resolution process.
Official level
Regular official level meetings are expected to provide a forum where any policy intentions or developments within the framework can be addressed. If and when officials become aware of potential issues or areas of disagreement, the first step set out in the framework is to seek to resolve this amongst officials without escalation.
The discussion with policy colleagues in each government to resolve the issue should:
determine the source of the disagreement;
examine evidence;
establish whether it is a significant concern and to work through possible solutions to the satisfaction of all parties.
Senior Official level
Where it has not been possible to resolve any disagreement at official level, this will initially be referred to Senior Officials for resolution.
At this stage Senior Officials can decide whether it would be appropriate to arrange a meeting with counterparts across governments.
After a solution has been agreed at official or senior official level, officials should produce risk assessment plans and a rationale for recommendation being made. Ministers then consider the recommendation individually and respond. In the event of agreement from all Ministers, the recommendation or approach is implemented.
Alternatively, Senior Officials may determine that the issue cannot be resolved at this stage and escalate the issue for discussion between Ministers.
Ministerial level
Ministerial level in this framework's dispute resolution mechanism refers to a level of escalation overseen by junior or senior ministers before the dispute is referred to intergovernmental structures (see Dispute resolution Ministerial level). Escalation to intergovernmental dispute resolution mechanisms is expected to be a last resort and only used when steps for resolving disagreements within the framework's Ministerial level is unsuccessful.
Any continuing disagreement, which cannot be resolved at official level in the ways set out above, will be referred to Portfolio Ministers for resolution. Ministers' consideration of the disagreement may be by inter-ministerial meetings or correspondence.
As set out by the framework, the making of legislation within the policy area may need to be postponed until all four governments are in agreement on how to proceed.
The parties may conclude, having considered potential impacts on patient safety, the JMC (EN) principles and the appropriate intergovernmental structures, that divergence is appropriate.
Dispute resolution Ministerial level
It is anticipated that recourse to resolution at Ministerial level will be as a last resort and only sought where dispute resolution at official level has failed. Disputes which reach Ministerial level will be resolved through intergovernmental dispute resolution mechanisms. Relevant intergovernmental disputes may concern the "interpretation of, or actions taken in relation to, matters governed by […] common framework agreements".
Intergovernmental dispute resolution mechanisms were considered as part of the joint review on intergovernmental relations. The conclusions of the joint review were published on 13 January 2022 and set out a new approach to intergovernmental relations, which the UK Government and devolved governments have agreed to work to. The joint review created a new three-tiered system for intergovernmental discussions, doing away with the old Joint Ministerial Committee structure.

The lowest and middle tiers have specific responsibilities for common frameworks. At the lowest tier, interministerial groups (IMGs) are responsible for particular policy areas, including common frameworks falling within them. At the middle-tier, the Interministerial Standing Committee (IMSC) is intended to provide oversight of the common frameworks programme.
The new IGR dispute resolution process follows on from the process at the official level. If a dispute cannot be resolved at the official level as set out in individual frameworks, it is escalated to the Ministerial level. The diagram below illustrates the general dispute resolution process for frameworks, including discussions between officials (square) and Ministers (triangle).i
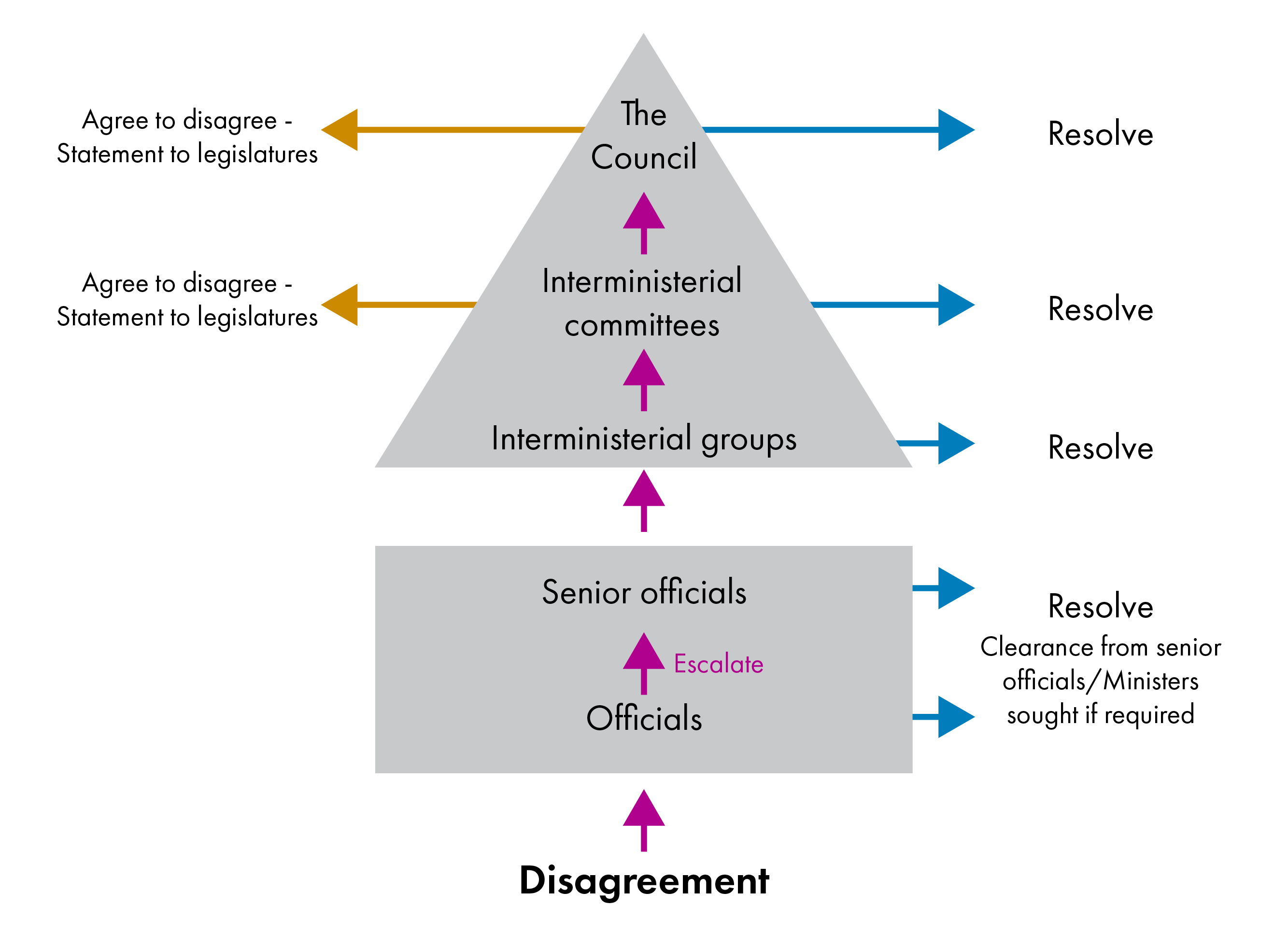
At the lowest level, interministerial groups comprising portfolio Ministers attempt to resolve the disagreement. If their attempts are unsuccessful, the issue can be escalated to an interministerial committee. If the interministerial committee is unsuccessful in resolving the issue, it can either agree to disagree, in which case each government makes a statement to their legislature to or escalate the dispute further. If a dispute is escalated to the highest level, third-party advice or mediation should normally be sought and made available to the Council. If the Council fails to find agreement, it is again required to make a statement to their legislatures.
The new process includes more extensive reporting requirements about disputes. The IGR secretariat is required to report on the outcome of disputes at the final escalation stage, including on any third-party advice received. Each government is also required to lay this report before its legislature.
The Office for the Internal Market (OIM) can provide expert, independent advice to the UK Government and devolved governments. Its advice and reports may, however, be used by governments as evidence during a dispute on a common framework.
Rachel Merelie of the OIM explained the position whilst giving evidence to the House of Lords Common Frameworks Scrutiny Committee in November 2021:
The OIM is not involved in dispute resolution. We are here to provide advice to government, using our economic and technical expertise…It is of course possible…that our reports are considered in some shape or form as evidence in support of that process, and we remain open to being used in that way.
Implementation
The framework was provisionally confirmed by the JMC(EN) and published on 23 March 2021. An updated version was published on 2 December 2021. UK legislatures now have the opportunity to scrutinise the framework and raise any concerns with their respective government. If scrutiny and any subsequent reappraisal of the framework leads to significant changes, the provisional framework may need to undergo further collective agreement before final confirmation. The framework will take effect once agreed by all parties and approved by Ministers from all four governments.
Framework Analysis
Current policy position
The draft framework relates to policy on the safety and quality of organs, tissues and cells, excluding reproductive tissue and cells.
This policy area was previously governed by harmonised EU Directives (EU Organs Directive (2010/53/EU) and the EU Tissues and Cells Directive (2004/23/EC)) and implemented in domestic legislation.
Broadly, the retained EU law in this area sets the quality and safety standards for organs, tissues and cells which includes:
the procurement, testing, processing, and storage of tissues and cells (including reproductive cells)
organ and donor characterisation, meaning the collection of the relevant information on the characteristics of the donor and the organ, including tissue typing tests, needed to evaluate suitability for organ donation and optimise organ allocation
transportation conditions of organs and distribution conditions of tissues and cells, including labelling and documentation
traceability requirements in respect of organs for transplantation and tissues such as corneas or bone and stem cells
notification requirements in the event of serious adverse events or reactions which may impact the quality and safety of organs, tissue and cells.
Maintaining a compatible minimum set of safety and quality standards between the UK countries will make it easier for organs, tissues and cells to continue to be shared across the UK.
Key issues
Under the UK/EU withdrawal agreement, Northern Ireland remains in the UK customs territory but remains aligned with EU regulations. If the rules around blood safety and tissues change in the EU, then Northern Ireland will have to realign with them, possibly leading to divergence from the rest of the UK.
The framework sets out the structures and processes for managing the impact of such changes should they occur. When questioned by the Health, Social Care and Sport Committee, the Minister for Public Health, Women's Health and Sport answered the following in a question about the probability of such divergence hindering the sharing of blood and tissues:
It is clear that, if there is a change in EU law, that will apply in Northern Ireland. That will need to be considered through the framework processes. The Scottish Government set out its view that, although we are not in the same situation as Northern Ireland, we are pretty keen to remain aligned with EU law where such alignment is appropriate and in Scotland’s best interests.
Health, Social Care and Sport Committee. (2022, February 1). Official Report. Retrieved from https://www.parliament.scot/chamber-and-committees/official-report/what-was-said-in-parliament/HSCS-01-02-2022?meeting=13568&iob=123130 [accessed 22 March 2022]
Scrutiny at other legislatures: House of Lords Common Frameworks Scrutiny Committee
The House of Lords Common Frameworks Scrutiny Committee are responsible for scrutinising and considering matters relating to all the Common Frameworks. It held an oral evidence session on 25 May 2021 to discuss the policy context of the Provisional Blood Safety and Quality Framework, the Provisional Organs, Tissues and Cells Framework and the Provisional Public Health Security framework. The stakeholders in attendance and providing evidence were:
Dr Julie Cavanagh, Convener, Faculty of Public Health in Scotland;
Mark Dayan, Policy Analyst and Head of Public Affairs, Nuffield Trust;
Ian Rees, Manager, Inspectorate Strategy and Innovation Unit, Medicines and Healthcare products Regulatory Agency (MHRA).
The House of Lords Common Frameworks Scrutiny Committee considered the Blood Safety and Quality, and Organs, Tissues and Cells (apart from embryos and gametes) Provisional Frameworks on 14 December 2021. In correspondence to the Minister of State for Health, Edward Argar MP, the committee noted:
"We are disappointed to note the absence in these frameworks of any commitments on ongoing engagement with Parliament. We note the absence of any commitments in the texts of these frameworks to publish reviews of the frameworks or to update legislatures on the outcomes of reviews. The Government has separately committed to improving transparency in Intergovernmental Relations. Transparency in this area should include regular statements to legislatures on the functioning of these frameworks.
We recommend that the frameworks should be updated to include a commitment to update the House of Lords, House of Commons and the three devolved legislatures on the ongoing functioning of these frameworks after the conclusion of the two-yearly reviews.
While we note the commitment to communicate changes in the frameworks to stakeholders, we regret the absence of a commitment to more meaningful ongoing stakeholder engagement."
In addition, the committee recommended:
We recommend that the first two-year review should include an open consultation process with stakeholders and the frameworks should be updated to include an ongoing commitment to stakeholder engagement where necessary.
House of Lords Common Frameworks Scrutiny Committee. (2021, December 14). Letter to Minister of State for Health, Edward Argar MP. Retrieved from https://committees.parliament.uk/publications/8242/documents/84234/default/ [accessed 20 December 2021]