Health and Sport Committee
Supply and demand for medicines
Executive Summary
In undertaking this inquiry into the supply and demand for medicines, we were anticipating exploring issues relating to the efficiency of the system and the levels of waste generated. However, in considering the themes raised with us a fundamental problem has become apparent - the system of supply and demand for medicines in Scotland does not have a focus on patients.
Instead, the system is burdened by market forces, public sector administrative bureaucracyi and reported under resourcingii, inconsistent leadership and a lack of comprehensive, strategic thinking and imaginationiii, allied to an almost complete absence of useable dataiii.
The lack of data collection and analysis on outcomes achieved via the prescriptions of medicines is of huge concern. The impact on individual patients of taking medicines is not being examined and worse, it is not routinely sought. Patients in primary care are not receiving follow up care to ensure the medicines prescribed were effective or even to ensure they were used.
We found the lack of care taken to understand people's experience of taking medicines impacted the system at every stage. We are clear that gathering, analysing and sharing this information in a comprehensive, systematic way across Scotland would be the single most beneficial action to result from this inquiry. This needs to be prioritised as a matter of urgency, which will require strong leadership.
Those we consider should have responsibility for solving problems or developing innovative solutions were often the very people who recounted the issues for us, but without accompanying ideas or impetus for changeiii. The vague statements we heard of the utility or need to do something without accompanying detail on how it would be achieved are ineffective in driving forward innovation and change, particularly in reference to data and evidence collectioniii.
Little detail was offered as to how change might actually be brought about, let alone at a pace proportionate to the prize to be gained. Nobody seemed willing or able to take on responsibility for driving forward the change many identified as essential. These issues are not new and we heard little by way of solutions and strategies from senior leaders within the health service about what would actually be done to address them at the pace and scale required. We propose energetic, imaginative, accountable, focused leadership is required in order to achieve the changes required. We strongly recommend the Scottish Government examine the lack of accountability for improvement allied to the reasons why leaders at all levels are not proposing innovative, coherent and comprehensive solutions which many told us would deliver efficiencies and savings.
Given their responsibility for developing a plan to implement the National health and wellbeing outcomes framework, we were surprised to hear little of the role of Integrated Joint Boards (IJBs) in more cost and clinically effective use of medicines. For bodies with such a fundamental role to be notable by their absence in this inquiry again suggests a lack of strategic oversight in this area.
The relationship between prescriber and patient was stark throughout the inquiry. The power of the prescriber to determine the clinical and cost effectiveness of a patient's treatment was described as almost absolute, and it is clear prescribers are instinctively reaching for the prescription pad, and are not taking the time to discuss medicines with patients to a degree that ensures the clinical and cost effectiveness of the prescription. Nor are comprehensive reviews of polypharmacy or long-term prescriptions taking place and there is nothing in place to monitor, evaluate or mandate this, resulting in at best waste and at worst harm to the patient. It also appears to us there is not a strict adherence to the principles of realistic medicine, patients are not equal partners in discussions on their treatment. One of the areas this is most exposed is social prescribing and prescriptions for non-medicine alternatives.
We believe the Scottish Government's contract with GPs is failing to mandate these behaviours and actions and must be revised. In the long term, without systematic changes the Scottish Government should consider whether the system of GPs being external contractors works well for patients. An approach which pays GPs from the public purse with no monitoring or evaluationvii of their actions is not acceptable.
The role of community pharmacists in dispensing medicines was clear and well defined, however we heard a wealth of evidence on the other benefits community pharmacists could bring in ensuring the cost and clinical effectiveness of medicines in Scotland. However, the lack of structure surrounding their relationships with both patients and the rest of the health services means they are failing to exploit their skills, knowledge and position for the benefit of patients. Discussions on whether and how medicines were taken, and the effects of these, are at best being recorded on Post It Notesiii and at worst disappearing without recordix. Patients expect and deserve a better system than this.
We are extremely disappointed that once again all roads lead to the dismal failure of the NHS in Scotland to implement comprehensive IT systems which maximise the use of patient data to provide a better serviceiii. Commentsiii on patients' surprise at how badly information is handled, particularly at junctions of care, were casually made, once again exposing attitudes not focused on patient care. Worse still, where a lack of patient focus was acknowledgediii, this was not followed by a solution or plan to take action, but simply left hanging for us to add to the list of issues with medicine management.
We again urge the Scottish Government to consider the IT and data requirements of the NHS across the country in a strategic way and design systems with long term utility as a matter of urgency. We recognise this is a large undertaking but similarly, we cannot keep concluding in reports as we have for the last 4 years that savings, efficiencies and above all better patient care are possible with modern IT, capable of data gathering, analysing and sharing, without demanding urgent action in this area.
Introduction
The Health and Sport Committee agreed to hold an inquiry into the supply and demand for medicines. This was in part based on the frequency of occasions the issue was raised with us by representatives of health boards and special health boards as part of our scrutiny of such bodies.
We agreed the inquiry would focus on four distinct areas—
Purchasing (including procurement and medicine price regulation, a reserved area undertaken at a UK level);
Prescribing (covering all licensed to write prescriptions);
Dispensing (covering hospital, pharmacy and GPs); and
Consumption (looking at effectiveness and wastage).
The overarching aim for our inquiry was to examine the management of the medicines budget, including the cost and clinical effectiveness of prescribing.
We also agreed that the purpose of the inquiry was not to consider the cost effectiveness of new medicines or whether particular medicines should be routinely available for prescribing by the NHS in Scotland. This issue arose in the written and oral evidence we received and we have limited our comments on these processes to their overall impact on the clinical and cost effectiveness of medicines throughout the system.
Engagement
We sought views from a wide range of bodies and individuals, and our call for submissions ran from September to November 2019. We requested comments on 4 areas—
Does the system ensure patients receive the most clinically and cost effective treatments and, if not, how can this be improved?
Does the NHS in Scotland achieve the most value from the money spent on medicines and, if not, how can this be improved?
In what ways can the system be made more efficient?
How can the medicines budget be controlled while maintaining clinical and cost effectiveness?
We received 58 written submissions. Further information was sought during our inquiry along with supplementary evidence from several witnesses who gave evidence. All correspondence and supplementary evidence can be found at Annexe B.
We are extremely grateful to all those who contributed to its inquiry, without the input we received, both written and oral, we would not have been able to undertake our work.
Structure of the report
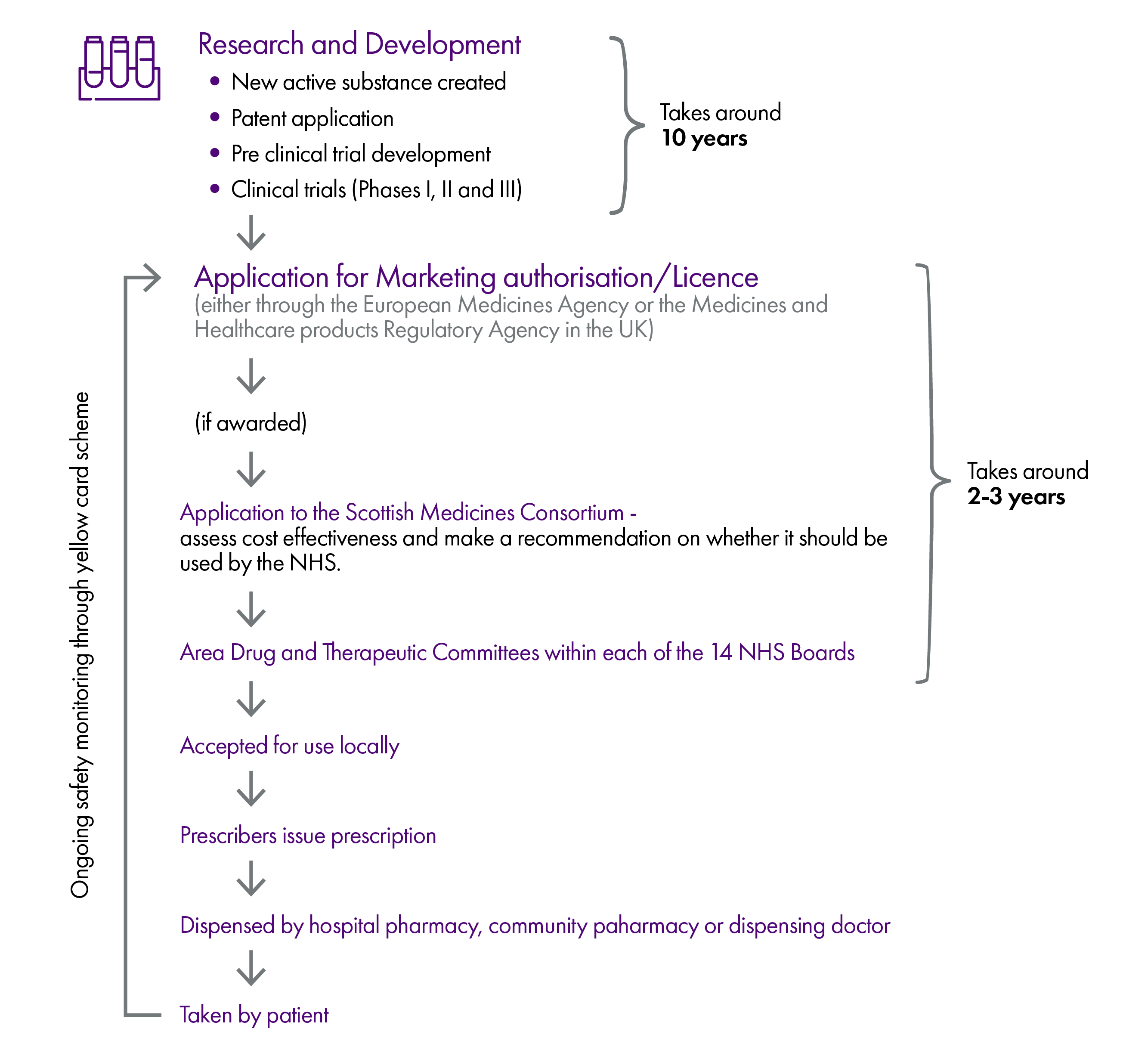
Our inquiry was broad and wide ranging, covering the supply and demand for medicines in Scotland from the research and development of a drug, to the consumption by a patient. The report reflects the distinct areas we have examined and received evidence on—
Licensing and acceptance for use in the NHS - this includes how consideration of new medicines by the European Medicines Agency, the Medicines and Healthcare products Regulatory Agency and the Scottish Medicines Consortium contributes or detracts from ensuring Scotland maximises spend on medicines
A glossary of terms and acronyms has also been provided at the end of the report.
Background
Our inquiry took place against the back drop of the most recent Audit Scotland report on spending in the NHS in Scotland, which notes "drugs costs have stabilised"i. The report states—
"The NHS in Scotland spent almost £1.8 billion on drugs in 2017/18, a reduction of 0.2 per cent in real terms since 2016/17. Good progress continues to be made in the proportion of generic medicines prescribed. This increased from 83.9 per cent in 2017/18 to 84.3 per cent in 2018/19."i
The net expenditure on medicines by the NHS in Scotland in 2017/18 was 16.6%, down 0.1% on the year before. The majority of spending on drugs takes place in community and family health services and this was again reduced from the previous year.
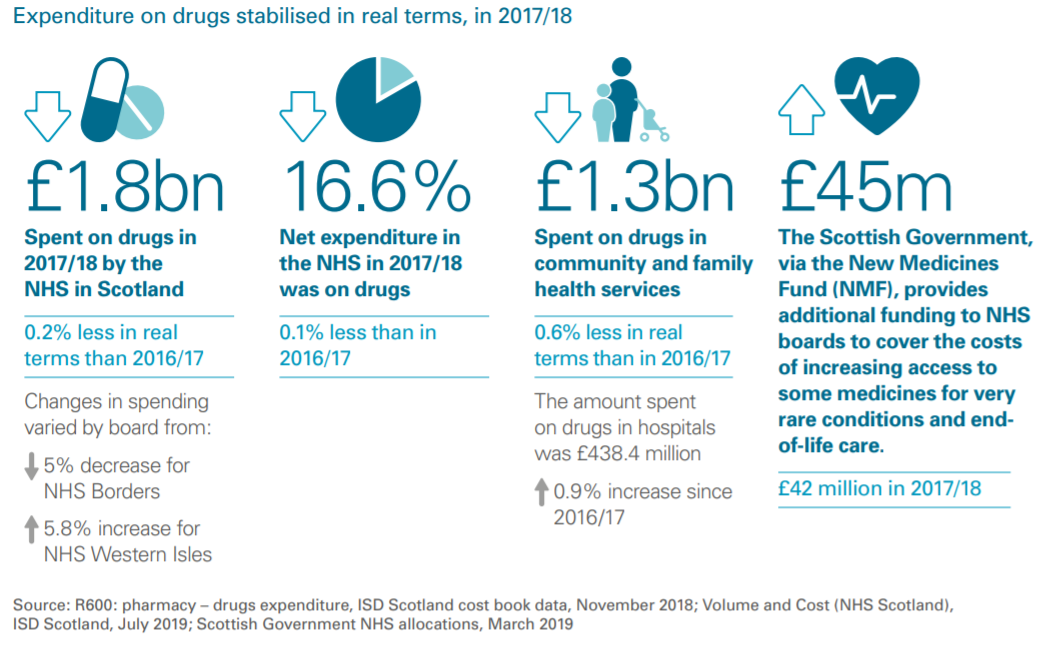
Stakeholders agreed medicines spend in Scotland is going in the right direction, with many telling us of the tightly controlled and meticulously scrutinised budget for spending on drugs, especially when compared to spending in other parts of the health service.
In relation to branded medicines, there are built in limits to spending increases as a result of the Voluntary Pricing and Access Scheme (VPAS) which it was noted provides a degree of predictability of spending. We were toldiii the UK compares favourably to other countries when it comes to prices paid for generic medicinesiii.
Research and development

We heard there was potential for the NHS in Scotland to achieve better value from medicines based on activity at the research, development and manufacturing phases of a medicine's existence.
These included—
Use of data from real-world experience of using medicines as opposed to reliance on evidence gathered during clinical trials; and
Preparedness for personalised medicines, including within manufacturing.
Each are explored in turn.
Real-world experience and clinical trials
Submissions we received suggested the evidence gathered in clinical trials, and on which Scottish Medicine Consortium (SMC) and National Institute for Health and Care Excellence (NICE) appraisals are based, did not always match the data collected based on real-world use of a medicine. Several reasons were cited for this—
The "eligibility criteria"i for clinical trials did not always match the groups to which a medicine was eventually prescribed;
Patients who eventually take medicines may have co-morbidities and this could affect the resultsii; and
Smaller populations were available to participate in the trials for some drugs, resulting in a lack of economic information required for SMC assessmentiii.
Celgene UKiii cited Cancer Research UK research showing clinical trials could prove a drug's safety and efficacy but did not always demonstrate the benefits to be derived for patients. They further noted "a greater emphasis on using real-world data of patients' treatment outcomes will be necessary to ensure, not only that outcomes for patients are personalised and optimal, but that the NHS can be confident it receives value for its medicines spend."iii
We recognise the value of knowing the wider benefits to be gained from medicines and the implications for clinical and cost effectiveness. As various stakeholders told us, medicines which are not having the desired effect are not good value for money.
Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS National Services Scotland, said—
"Having that real-world evidence over time could greatly help us to evaluate what [medicine] is the most cost effective, once there is experience of using the medicine. However, to do that, we need much more efficient IT systems to collect outcomes data. As we have already heard, there are some great initiatives, such as the cancer medicines optimisation programme, but it is still early days and there is more work to be done."ii
The data on real-world application of medicines is important to both manufacturers and the NHS alike in terms of the value of medicines. As stated above, medicines which are not having the desired effect are not good value for money. This is a theme explored further in the VALUE AND OUTCOME BASED PRICING part of our report.
Personalised medicine
Personalised medicine involves tailoring drugs and treatment to the specific requirements of an individual's version of an illness and their biology.
The benefits include—
Increased efficacy of a drug (and therefore better value);
Reduced adverse reactions (and therefore better clinical effectiveness); and
Reduced waste.
Several themes were pursued in evidence on personalised medicine—
Waste;
Manufacturing technology; and
Preparedness for personalised medicine in the healthcare system.
Waste
Several representations, including the Chief Pharmaceutical Officeri and the University of Strathclydeii, noted the potential for personalised medicine to reduce or even eradicate waste of medicines by ensuring maximum tailoring to the specific need of the patient.
Manufacturing technology
Alison Culpan of the Association of the British Pharmaceutical Industry (ABPI) suggested data enabling the identification of patients who could benefit from innovations in gene therapy was lacking, and this was squandering the opportunity to get the "right outcomes and value for money from what is spent on medicines."i
The University of Strathclyde stated manufacturers were reliant on outdated technology and called for new production and supply chain technologies to "revolutionalise healthcare delivery"ii. However, they said such a project was unlikely to be achieved by one company or group acting independently and called for cross sector and Government intervention.
Preparedness for personalised medicine in the healthcare system
Although it was clear to us the technology is in its infancy, a range of witnesses spoke of the need for current systems to adapt to the medicines of the future.
We received several representations urging the SMC to consider future proofing assessment processes. The Blood Cancer Alliancei welcomed approvals of chimeric antigen receptor T-cell (CAR T cell therapyi) but said challenges lay ahead in terms of greater demand and therefore budget impact. They called for further work with industry to "scope the likely challenges and financial impacts of these innovative treatments."i
It was noted personalised medicines would represent higher costs in some circumstances but this should be assessed and balanced against the likely benefits. There were calls for reform of the SMC assessment to accommodate personalised medicine and for a review similar to the work NICE are carrying out in this area.
Dr Alan MacDonald, Chair of the SMC, informediv us about the SMC's horizon scanning activities (discussed further in the SCOTTISH MEDICINE CONSORTIUM HORIZON SCANNING section of this report) and preparations for the next generations of medicines. The ABPI noted the substantial evidence which would be required by the SMC for advanced therapies, indicating "the NHS needs to balance the risk of treatment failure with a manufacturer's need to recoup the enormous cost associated with development."i
Technology in manufacturing was another area where stakeholders felt systems would require to be updated to accommodate innovations.
We note and welcome the ambitions in the area of personalised medicine described by the Chief Pharmaceutical Officer. We seek from the Scottish Government specific detail as to—
Which areas it currently considers require adaptation to embrace personalised medicine;
How this will link with other data and technology projects such as the National Digital Platform; and
Budgetary planning for the accommodation of personalised medicines within the health and social care service in Scotland.
We encourage the Scottish Government to consider the technology used by manufacturers and wholesalers in the creation and delivery of personalised medicines and consider what further incentives and support could be provided to develop these.
We recommend the Scottish Government make provision for the use of personalise medicine in the future, including data collection (a theme considered in detail in the DATA and IT section of this report).
Research and Development - conclusions
On the use of real-world data rather than clinical trials, we share the enthusiasm of stakeholders for better utilisation of such information. We also share their views on the data infrastructure required to achieve a system for proactively and comprehensively gathering this information.
The advent of personalised medicines is an exciting prospect in terms of cost and clinical effectiveness according to many who spoke to us. Above all, the potential for personalised medicine to achieve positive impacts on budgets and waste, and improved clinical outcomes for patients, is clear. This is a theme we return to throughout the report.
We believe the Scottish Government should be doing all it can to support the use of personalised medicine, and encourage and support innovation in Scotland, while remaining mindful of the need to have in place appropriate systems and means of tracking and measuring outcomes.
However, we are clear such a development requires to be properly planned in terms of assessment processes, data and technology and budget required. In addition, we would expect cost implications along with measurement of effectiveness to be included in processes at the outset.
Licensing and acceptance for use in the NHS

The following sections cover how medicines are licensed and accepted for use within the NHS in Scotland. Products must first obtain a licence or marketing authorisation, before being assessed by the Scottish Medicines Consortium (SMC) for use within the NHS in Scotland.
The impact of these processes on clinical and cost effectiveness was the subject of various representations to us as part of our inquiry.
Licensing
The Scottish Parliament Information Centre has described the licensing of drugs in the UK and the role of the Medicines and Healthcare products Regulatory Agency (MHRA)—
"Before any medicine can be used in the UK, it usually has to receive a marketing authorisation from either the Medicines and Healthcare products Regulatory Agency (MHRA), or the European Medicines Agency (EMA). The MHRA is the UK's licensing and regulatory authority and its functions are reserved to Westminster. It therefore operates on behalf of the 4 UK countries. A marketing authorisation is often referred to as a 'licence' and can only be issued following the completion of clinical trials that show—
- the medicine treats the condition it was developed for;
- the medicine meets safety and quality standards; and
- the medicine does not cause unacceptable side effects.
At the moment there are a number of routes for a medicine to obtain a marketing authorisation in the UK but at a very basic level this boils down to either applying via the EMA, or applying to the MHRA"i
We were particularly interested in two specific aspects of licensing and their impact on the use of medicines in Scotland—
Licensing for more than one indication; and
Evidence provided by pharmaceutical companies to support licensing.
Licensing for more than one indication
We were interested in medicines which could have alternative uses to their original design and how this could achieve both savings and better results for patients (a current example which has arisen since our evidence sessions being the use of Remdesivir, an anti-viral drug, to shorten the recovery time from COVID-19). Given the complexities described to us, we were also keen to understand how applications could be encouraged for licences for existing medicines which could have an impact on conditions beyond that which they were originally designed for.
Representatives of the pharmaceutical industry suggested there was work going on from their perspective as to how this could be progressed.
Warwick Smith, Director General, British Generic Manufacturers Association (BGMA), discussed the scope for looking "at older medicines that can be repurposed for new uses"ii. He described discussions as "well advanced"ii on this. Alison Culpan of the Association of the British Pharmaceutical Industry (ABPI) saidii it was hard to quantify how much a licence application for an additional indication may cost. She providedv detail on recommendations of an Association of Medical Research Charities reportvi on ways to incentivise repurposingvii, including research and development tax credits, creation of a fund, prescribing resources and support for clinicians. It also covered ways to enable the MHRA to communicate more easily with those seeking to research repurposing and for including repurposed medicines in the Accelerated Access Pathway.
She also highlighted work undertaken on this issue by the European Commission Expert Group on Safe and Timely Access to Medicines for Patients (STAMP). She said the ABPI had been working on a draft framework with the Commission—
"This framework operates by improving communication between academia (who are often the first to identify medicines for repurposing), the regulator and industry partners. The process would enable an academic to contact the regulator for advice around the eligibility of a molecule, this advice would then enable the “academic champion” to take this project to an industry partner who can subsequently evaluate the risks and opportunities involved. A lack of awareness and communication between academia, the regulator and industry is often the first and largest barrier which prevents older drugs from being repurposed. This framework provides a template to de-risk and improve the process."v
We were told of circumstances in which medicines were being used for indications for which they were not licensed and Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS National Services Scotland, spokeii of legal challenges in this regard.
We were interested in whether there was a role for an organisation to encourage pharmaceutical companies to apply for licences for different indications for existing drugs. Both the MHRAii and the SMCxi told us they could have no role in encouraging applications. Dr Alan MacDonald, Chair of the SMC, added—
"In certain circumstances HIS [Healthcare Improvement Scotland] can advise on the off-label/unlicensed use of cancer medicines. HIS hosts an Off-label Cancer Medicines Programme recently commissioned by Scottish Government’s Cancer Division in response to its Beating Cancer: Ambition and Action strategy. This programme aims to provide advice to NHS Board Area Drug and Therapeutics Committees on the managed entry of off-label uses of cancer medicines. Through this advice the programme will facilitate equity of access to off-label uses of cancer medicines and reduce unwarranted variation for patients across NHSScotland. This programme is expected to deliver the first piece of advice to NHSScotland in summer 2020."xi
Rose Marie Parr, Chief Pharmaceutical Officer, said the Scottish Government encouraged pharmaceutical companies to assess their licence. She added—
"We see some older medicines being repurposed and used for very different things. There needs to be some consideration of that huge burden on some parts of pharmaceutical industry to get their medicines to licence—that is very costly and takes time. Some of those processes could be shortened a bit, which would help."ii
Alison Strath, Principal Pharmaceutical Officer, Scottish Government, added "...we would need to work to understand what levers there might be to support repurposing"ii and although the Cabinet Secretary for Health and Sport, Jeane Freeman, foresaw challenges, she did not consider these to be "insurmountable".ii
We request the Scottish Government provide details of what plans it has to respond to the suggestions provided by the Association of the British Pharmaceutical Industry (ABPI) on how applications for additional licences for medicines can be encouraged and incentivised.
We recommend the Scottish Government works with the UK Government and the MHRA to streamline and shorten the processes it described to us as causing barriers to pharmaceutical companies applying for additional licences and health care technology assessments for new indications for existing drugs.
We encourage the Scottish Government to provide details of how it will encourage pharmaceutical companies to apply for new licences for new indications for previously licensed medicines, including how timescales can be shortened to assist with this and the other levers proposed by the Scottish Government.
We also request the Scottish Government give consideration to commissioning and funding clinical trials on medicines for use in conditions of significant public health impact or where there is a significant lack of cost effective treatments.
Adaptive licensing
Alison Culpan, Scotland Director of the ABPI, highlightedi the issue of adaptive licensing, which she described as "emerging"—
"Adaptive licensing predominantly applies to new treatments where ‘drug candidates’ that meet a serious unmet medical need can be initially approved for use in a restricted patient group. This is then gradually expanded to broader patient populations as additional safety and efficacy data is generated. Whilst this doesn’t apply to older molecules or those in limited indications, it does demonstrate that flexibility is required to ensure patients continue to benefit from fast access to new medicines."i
We request details of whether adaptive licensing can be applied to new indications for existing drugs and the role the Scottish Government can play in encouraging this.
Evidence in support of licence applications
Jonathan Mogford, Director of Policy, MHRA explainedi the process by which evidence to support licence applications was peer reviewed. He said the licensing process was "part of the important interaction with companies that ensures that the claims that are made for products are backed by clear scientific evidence"i and notedi the agency's enthusiasm for the debate about using "real-world" evidence (please refer to the REAL-WORLD EXPERIENCE AND CLINICAL TRIALS section of this report) and suggested improved data on outcomes would fuel this further (this issue is considered fully in the OUTCOMES section of this report).
In correspondence with us, he further outlined the basis of decisions on evidence—
"All decisions on approval of medicines, wherever these are taken, are based on satisfactory demonstration by the applicant of the safety, quality and efficacy of new medicines. In the UK, the decisions are informed by independent advice from the Commission on Human Medicines. The MHRA does not plan to change the evidence standards for approval of new medicines and will use existing flexibilities, such as conditional approval and incentives for rare diseases to ensure that unmet clinical needs can be met by licensing of new products."iv
Dr Alan Macdonald, Chair of the SMC, outlined how the organisation treated medicines with less supporting evidence than they might otherwise expect and said "...if a medicine that is licensed by the EMA comes to us, we have to assume that it has been shown that the benefits outweigh the risks. However, our job is different; it is to say whether the benefits justify the cost."i
Licensing - conclusions
The opportunities presented by licensing medicines for more than one indication are currently being missed in our view, due to barriers described to us by stakeholders. We have recommended the Scottish Government consider how it can better encourage trials and expedite licensing for additional indications of existing drugs to maximise their use within the NHS.
On the standard of evidence required for new medicines, we are satisfied the processes are working although refer to our previous conclusion better use could be made of 'real-world' experience of using medicines, rather than relying solely on clinical trial data.
Access to new medicines
While the subject of access to new medicines does not form the focus of our inquiryi, many of the submissions received reflected on how new medicines are assessed in Scotland. Issues raised by work by the previous Health and Sport Committee in its session 4 inquiryi on Access to New Medicines are relevant to this area.
In the period since that inquiry was undertaken, an independent review was held "to assess the impact of the new approach introduced in 2014 by [Scottish Medicines Consortium] SMC" and it reportediii to the Scottish Government in December 2016. The Scottish Government provided us with updates on progress towards delivery of the recommendations in the review in November 2017iv, February 2018v, May 2018vi, June 2018vii and 13 January 2020viii.
This section will consider evidence received on the following themes
Scottish Medicine Consortium (SMC) Assessments;
Patient Access Schemes;
Evidence Based Approvals; and
Interaction with the Pharmaceutical industry.
Scottish Medicines Consortium (SMC) assessments
The SMC is a branch of Healthcare Improvement Scotland (HIS) which carries out assessments on all new medicines for use in Scotland. It consists of a committee of "clinicians, pharmacists, NHS board representatives, the pharmaceutical industry and the public". It also has a horizon scanning function, which according to HIS "aims to support health boards with financial and service planning for new medicines in the pipeline that may be accepted into routine clinical use."ii As previously indicated, its primary role is to say whether the benefits of a new medicine justify the costs.
The following diagram describes the process by which drugs are assessed—
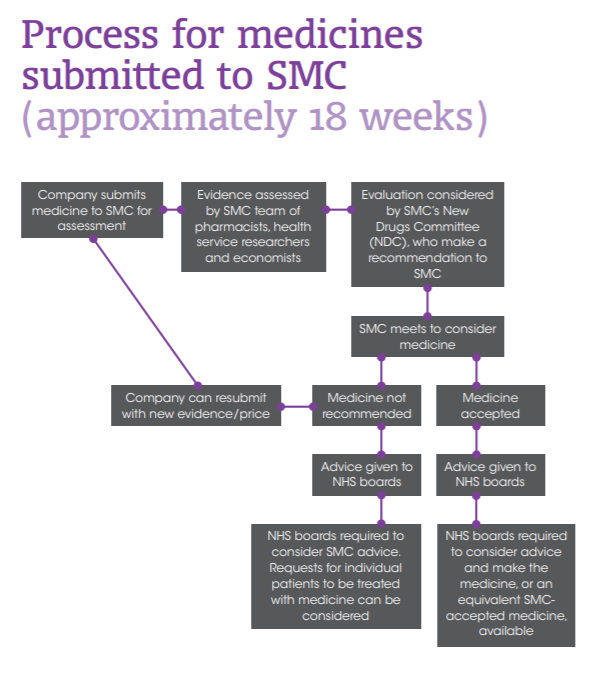
Many, such as Celgeneii and the British Medical Association (BMA)ii praised the efficacy of the assessment processes of the SMC and suggested these kept the budget under control while also allowing for access to new medicines. The Association of the British Pharmaceutical Industry (ABPI) saidii the healthcare technology assessment conducted by the SMC was a "leader" in balancing cost and medical value, and said it made Scotland an attractive place for manufacturers to test new products.
The Royal College of Physicians of Edinburghii and others highlighted the potential waste from not using the most clinical and cost effective medicines available, stating the prescription of drugs of limited efficacy could "adversely apply pressure on the use of existing drugs of very good cost effectiveness, and on other treatments which provide better cost effectiveness". The difficulties in assessing cost effectiveness of medicines are discussed in the VALUE section of this report.
While many heralded the SMC assessment process and said their approval status inferred clinical and cost effectiveness, a number of questions were also raised.
We considered the following themes presented to us in evidence on the assessment processes of the SMC—
Speed of the assessment process compared with the rest of the UK;
Changes since the Review of Access to New Medicinesvii (the Montgomery Review), including—
Review of assessment methods;
Peer Approved Clinical System (PACS) Tier 2;
Patient and Clinician Engagement (PACE);
Clinical and cost effectiveness of new medicines;
Access to end of life, orphan and ultra-orphan medicines; and
Interim acceptance.
The assessment of value; and
Horizon scanning.
Speed of assessment compared with the rest of the UK
Merck, Sharp and Dohmei, Pfizer UKi, the Blood Cancer Alliancei and Rocheiv suggested SMC assessments were slower than UK counterparts, thus denying availability of medicines to patients and the cost benefits to the NHS for longer.
Others such as Prostate Cancer UKi expressed their concerns about delays and Jansseni were among those who called for investment of "money and resource" to allow the SMC to deal with delays in assessment.
While noting the issues raised with us were predominantly from bodies with an interest in seeing medicines becoming available quickly, we would welcome the view of the Scottish Government as to the reasons for potential differences in assessment speeds throughout the UK and what, if any, action is required by the Government or the SMC to rectify this.
Assessment of licensed medicines for cost effectiveness
We note the process of approval by the SMC starts with companies submitting medicines for assessment. The MS Society Scotlandi suggested there should be a more effective system for accessing medicines which were licensed but had not yet been assessed by the SMC in order to ensure patients can access the most effective treatments. We concur with this view.
We recommend the Scottish Government work with the SMC to develop a more effective system for accessing medicines which have not been submitted for assessment to the SMC based on the premise every newly licensed medicine should be assessed for cost effectiveness regardless of whether the manufacturer applies.
Changes since the Montgomery Review - review of assessment methods
In the Review of Access to New Medicines, Dr Brian Montgomery, Chair of the Review, wrote "...the overwhelming sense encountered was that there was a high level of satisfaction with SMC and that its processes remain robust."i
Dr Alan MacDonald, Chair of the SMC, said—
"The process has changed to allow greater access to medicines for cancer and rare conditions, which was a specific policy intent."ii
Nonetheless, there were several areas highlighted to us where stakeholders felt improvements could go further—
The BMA impliediii there is still an imbalance in the system based on the difficulties in prescribing off-formulary or unlicensed medicines contrasted with the high approvals for drugs approved at speed for small groups of patients;
The ABPIiii , Jansseniii and the Blood Cancer Alliancevi suggested assessments for combination therapies should be considered, noting the difficulty in demonstrating value and the financial barriers to companies to bringing these forward for assessment, meaning patients were missing out on clinically effective treatments. The ABPI also said the SMC should maintain an awareness of other assessment bodies' approach to consideration of combination therapies;
Roche Products Ltd said "Whilst robust, Roche believes that current methods of value assessment are becoming restrictive and patients in Scotland are not getting access to some clinically and cost-effective medicines when compared to the rest of the UK. Evolution of process must be seen as a priority to ensure delivery of best value and equitable access to cost-effective treatments across the UK."iii They suggested further reforms to Patient Access Schemes and Genomic Profiling.
Merck Sharp and Dohme noteiii a lack of commitment to review assessment processes for specialised medicines for which the quality adjusted life year (QALY) and Institute for Clinical and Economic Review (ICER) measures are not appropriate, resulting they say in patients missing out on medicines for specialised conditions. They believed such assessments were not suitable for every medicine.
Cancer Research UK suggested assessment of clinical and cost effectiveness was becoming more challenging and new "commercial and commissioning approaches are required in response to this trend, to ensure effective management of the medicines budget".iii
When questioned, Dr Alan MacDonald, Chair of the SMC, defendedii the "rigour" of the SMC appraisals process, acknowledging there may still be debate around access levels. He describedii the SMC function of comprehensive consideration of new medicines as compared with existing options as unique. Dr Brian Montgomery outlinedii his views on how changes implemented since his review of access to new medicines in Scotland had ensured the SMC had more flexibility which meant less circumvention of the process for patients to gain access to new medicines, therefore improving the robustness of the SMC appraisal system.
The BMAiii, the Royal College of Physicians of Edinburghiii and the Royal College of GPsiii were critical of the approval of medicines following political pressure and the importance of evidence based assessments and prescribing was emphasised by many throughout the inquiry.
Changes since the Montgomery Review - Peer Approved Clinical System (PACS) Tier 2
While the SMC assesses and takes decisions on medicines for routine use in the NHS as a whole, there are routes for patients and clinicians to access rarer medicines. Prior to the Review of Access to New Medicinesi, the Peer Approved Clinical System (PACS) and the Individual Patient Treatment Request (ITPR) systems were available.
The review recommended the Scottish Government should—
"Review and evaluate the experience of PACS to date with a view to deciding on any required modifications and thereafter agree the final model and timescales for implementation in NHSScotland."i
In response to this, a review of PACS Tier 1 was undertaken and a second tier was developed to replace the ITPR process. In June 2018, the Peer Approved Clinical System (PACS) Tier 2 was introduced to provide a new route for patients to access rarer medicines.
The advent of PACS Tier 2 was welcomed by respondents to our call for evidence such as Celgeneiii, who said it strengthened the system, Cancer Research UKiii and Pfizer UKiii.
NHS Fife Area Drug and Therapeutic Committee (ADTC)iii, NHS Lothian ADTCiii and NHS Bordersiii said PACS removed a health board's ability to consider cost or affordability where the SMC had already deemed a medicine not to be cost effective and more patients would access them as a result (as PACS facilitates access and does not allow boards to consider the cost). We sought the view of the Cabinet Secretary for Health and Sport on this issue in correspondenceix and also her view of when the second stage of the review of PACS would be completed.
However, others identified areas for further consideration including consistency of application and review of PACS Tier 2.
Changes since the Montgomery Review - Patient and Clinician Engagement (PACE)
Celgenei were supportive of the Patient and Clinician Engagement (PACE) process and the Cabinet Secretary for Health and Sport, Jeane Freeman, informed us of both the fulfilment of the recommendations on PACE but also of the next steps to "explore how PACE actually shapes decision-making"ii.
We welcome this work and look forward to receiving details of developments.
Changes since the Montgomery Review - Clinical and cost effectiveness of new medicines
While recognising the welcome outcome of increased access to new medicines as a result of changes implemented since the Review of Access to New Medicines, we were disappointed to hear some thought this was at the expense of clinical and cost effectiveness.
The Royal College of GPs expressedi the view changes in policy had seen medicines approved which may not have been previously and said "the clinical effectiveness of new medicines as a result may not be to the same level as they were historically". NHS Grampian ADTCi said medicines were approved with less evidence of clinical or cost effectiveness than would previously have been the case while Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clyde said "Through the changes to the policy position in Scotland, boards are now making available medicines that we would consider to be no longer cost effective. That has an impact in that it diverts resources away from other interventions that we might consider to be cost effective."iii
The views of NHS Grampian ADTC and Gail Caldwell provide an example of a frustration experienced throughout this inquiry on the role of NHS leaders, although we sympathise with the predicament.
If an ADTC does not have control over items for inclusion on a formulary, what is their purpose? Senior pharmacists we heard from throughout the inquiry helpfully identified a number of issues within health boards for us, but provided no solutions to these. If a Director of Pharmacy is not in a position or empowered to ensure resources are being applied to the most effective interventions, this is a larger problem than ineffective medicine prescription.
We recommend the Scottish Government consider the role of health boards within PACS decision making processes to make clear what the role of ADTCs, directors of pharmacy and other senior leaders is.
Changes since the Montgomery Review - access to end-of-life, orphan and ultra-orphan medicines
Rarer medicines defined as end-of-life, orphan and ultra-orphan can be defined as follows—
| End-of-Life Medicine | "A medicine used to treat a condition at a stage that usually leads to death within 3 years with currently available treatments."i |
| Orphan Medicine | "A medicine with European Medicines Agency (EMA) designated orphan status (i.e. conditions affecting fewer than 2,500 people in a population of 5 million) or a medicine to treat an equivalent size of populations irrespective of whether it has designated orphan status."i |
| Ultra-Orphan Medicine | "To be considered as an ultra-orphan medicine all criteria listed should be met—
|
Recommendations 6 and 7 in the Review of Access to New Medicines were—
"Review the definitions for end-of-life, orphan and ultra-orphan medicines to ensure that the definitions used remain suitable to deal with the assessment of anticipated new treatments such as targeted medicines, increasing use of combination therapies and the impact of genomics.
Develop, agree and implement a new definition of “true ultra-orphan medicine” to take account of low-volume, high-cost medicines for very rare conditions."i
Recommendation 11 called on the Scottish Government to "Develop and implement a new assessment and approval pathway for true ultra-orphan medicines that restricts the role of SMC to health technology assessment and places the responsibility for the final decision on availability elsewhere."i
In January 2020, the Cabinet Secretary informedvi us all three recommendations had been implemented. While the definitions for end-of-life and orphan medicines were deemed suitable, a new definition for ultra-orphan medicines was devised. As of January 2020, the new definition had tested "more than twenty medicines".vi We were interested in whether new definitions had posed any issues thus far and the Cabinet Secretary said positive feedback had been received by pharmaceutical companies. This tallied with responses to our call for views, with organisations such as Celgene praising the strength and adaptiveness of Scotland's healthcare technology assessment processes, which it said "are ensuring Scotland receives good value for the money it spends"viii.
However, others were reserved about the success of the new processes, with companies such as Chiesi Ltd saying it was "early days"ix for the new pathway. The European Medicines Group called for "similar energy to be put behind access to medicines for common conditions as well as orphan and end-of-life conditions".viii
We recognise it is still early days for the new definitions and pathways, and request the Scottish Government publish reports on evaluations.
Changes since the Montgomery Review - interim acceptance

The SMC said it was working on extending interim acceptance to more new medicines. The notion of conditional approval was welcomed by stakeholders including the European Medicines Groupi and we were told of several areas where the new interim acceptance would be beneficial.
Dr Alan MacDonald, Chair of the SMC, said the debate on outcome based pricing (see the VALUE AND OUTCOME BASED PRICING section for further analysis) would be one the SMC would have to consider as its interim acceptance option became more "mature"ii, noting there was a grey area for medicines which worked, but not quite as well as originally claimed.
The ABPIi described challenges ahead for the SMC, including evidence from trials based on decreasing participant samples, and suggested expansion of the number of medicines accepted under the "interim acceptance" status could help. Bristol-Myers Squibbi said this route should be available for a "wider group of medicines not just those with conditional marketing authorisation."
The Cabinet Secretary for Health and Sport told us the SMC was "....now exploring extending the use of the conditional acceptance option, specifically in the context of clinical uncertainty whether further data is likely to [be] forthcoming at a relatively early stage as a consequence of ongoing clinical trials."v
Value
The concept of value was highlighted to us in several written submissions. Stakeholders suggested this was a subjective assessment and could have multiple meanings - what is good value for an individual may not be good value for the population. It was also argued the value of a medicine could change depending on its age or its successors. The question of the efficacy of a cheaper medicine and whether this was worth the spend in comparison to better clinical results from more expensive medicines was also raised.
Matt Barclay of Community Pharmacy Scotland said "The cost of medicines should also be seen in terms of value, rather than in terms only of cost. When they are used appropriately, most medical interventions using medicines are perfectly good value for money. The situation has to be looked at in that light."i Similar points were made by a number of pharmaceutical manufacturers.
Prostate Cancer UKii were among those who asserted the use of medicines which may carry a high price tag, but which achieve huge savings in other forms of treatment, should be considered good value. The European Medicine Groupii and Healthcare Improvement Scotland (HIS)ii suggested medicines spend should not be considered in isolation from the health and social care budget as it risked discounting savings to be achieved across a pathway. Pfizer UKii also suggested wider societal costs, including to the economy, should be accounted for when considering the value of medicines, while the MS Society Scotlandii called for consideration of the impact on the welfare budget. HIS noted the "cost effectiveness analysis to drive value for money is not used uniformly across all areas of medicines spend."ii and the Cabinet Secretary for Health and Sport, Jeane Freemani, suggested evidence gathering in this area was hard and there was not a Scotland wide approach.
Many believed the SMC processes achieve good value for money and praised the system.
Scottish Medicine Consortium horizon scanning
The Scottish Medicines Consortium (SMC) described its horizon scanning functions as—
"SMC provides Health Boards with early intelligence on new medicines in development to support their financial and service planning. SMC is involved in the use and development of UK PharmaScan, a UK-wide horizon scanning database that provides information on new medicines, indications and formulations in the pharmaceutical pipeline. Forward Look, the SMC’s annual horizon scanning report, is published in October each year, it provides concise information on new medicines/indications that are expected to impact Boards over the following financial year. The report is accompanied by a financial planning tool describing estimated uptake and potential budget impact. SMC provides biannual updates to allow Boards to adjust their financial plans within year. We know that Forward Look reports are highly valued by Boards."i
In terms of recent activity to prepare for the next generation of medicines, the SMC told us it had issued briefings recently on "advanced therapy medicinal products and medicines with histology-independent indications" and anticipated more personalised medicines for patients with specific biomarkers, regenerative cell therapies and "complex combination regimes for cancer medicines"i.
We were interested in whether the horizon scanning function of the SMC was working effectively.
Dr Scott Jamieson of the Royal College of GPsiii posed the question of whether the SMC was assessing medicines against the qualities of value to patients and suggested it was not. David Coulson, Assistant Director of Pharmacy, NHS Tayside iii said the SMC's work was vital to ensure there were no surprises and emphasised the importance of a good evidence based to ensure value from medicines. Dr Jamiesoniii also stressed the need to collect good outcomes data to achieve this (see the OUTCOMES section of this report for further detail).
Many stakeholders believed the SMC processes for horizon scanning would have to be updated to accommodate personalised medicines (discussed fully in the PREPAREDNESS FOR PERSONALISED MEDICINE IN THE HEALTHCARE SYSTEM section of this report). Rochevi called for a consultation, akin to a review undertaken by the National Institute for Health and Care Excellence (NICE), on SMC processes and preparedness for assessing personalised medicine and updated horizon scanning. Pfizer UKvi suggested there was room for ongoing review in this area also to ensure patients could gain early access to new innovations.
The Cabinet Secretary for Health and Sport, Jeane Freeman, said—
"The new medicines fund is vital, but we need to parallel the work that the SMC is doing in considering its processes in anticipation of precision medicine and coming innovations. We need to consider how we fund and whether we should alter our approach in the light of innovative and important developments for patients."iii
We request detail of how and the planned timescale in which the Scottish Medicines Consortium processes will be reviewed and updated to accommodate innovations in medicines.
We recommend the Scottish Government review how it intends to fund this work, as well as the innovative medicines of the future to ensure Scottish patients are world leaders in receiving the best possible pharmaceutical treatment.
SMC assessment processes - conclusions
The healthcare technology assessments of the SMC were lauded as world leaders and the processes, as well as recent updates to these, were welcomed in the main for their contribution to the clinical and cost effectiveness of medicines used by the NHS in Scotland. There was general consensus improvements had been achieved through the Review of Access to New Medicines, but that the programme of reform and review must continue to ensure processes remain able to accommodate innovation.
There were calls for updates to assessment methods and measures to speed up assessments and we suggest the Scottish Government and the Scottish Medicines Consortium consider these suggestions. We also look forward to seeing how measures such as interim acceptance and the new definitions of end-of-life, orphan and ultra-orphan medicines progress.
Finally, we are in full agreement with evidence suggesting the value of medicines should be considered as well as the cost, and the full range of benefits and savings medicines could provide, both in health and across the economy, should be accounted for in decisions (see the VALUE section of this report for further detail).
Patient Access Schemes

According to Jansseni, 54% of the medicines for which the Scottish Medicines Consortium (SMC) published review advice between 1 January 2018 and 31 October 2019 had a Patient Access Scheme (PAS) discount and Celgenei noted a steady increase in their use also. Patient Access Schemes are used to discount the cost of new drugs in the early years of their use.
Roche Products Ltd proposed revisions could be made to the criteria for PAS to "drive further value"i and Pfizer UK called for revisions to the system.
The Blood Cancer Alliance noted two types of PAS are available: a simple scheme and a complex schemeiv. The group called for a more "flexible and transparent commercial framework"i, referring to the use of complex discount schemes and the difficulties these could bring. The group called for the guidance to be "revised to deliver a more mature, flexible and transparent framework for commercial discussion, focused on overcoming identifiable barriers to access and incentivising early engagement and flexibility on the part of industry."i
The Blood Cancer Alliancei described the dichotomy between the commercial confidentiality of the schemes and the need for transparency in decision making for the public, noting the former helped ensure good value for the NHS. They further added—
"In Scotland a further complexity exists in that some treatments do not have a formally approved PAS, although they are reimbursed by NHS Scotland at a discounted price. Where this is the case, the SMC will not take into account the discount price for the purpose of assessing the cost effectiveness and instead apply the full list price. This means that cost effectiveness decisions are not being based on the true cost of the combination to NHS Scotland. This is leading to inefficient decisions."i
Bristol-Myers Squibb said—
"Any scheme not presented as a simple discount is regarded as complex and PASAG assessment is made with their established view that these schemes introduce cumulative administrative burden for the NHS and that their perceived financial benefits may not be fully realised in practice. They therefore tend to be rejected. The Committee should investigate the proportion of SMC submissions that include a PAS, what proportion are regarded as simple or complex and what proportion of complex PAS are accepted."i
We recommend the Scottish Government should review, along with the Scottish Medicines Consortium and the Patient Access Scheme Assessment Group, how the actual cost to be charged to the NHS on approval of medicines can be "assessed in the absence of a formal [Patient Access Scheme] PAS"i.
We would welcome the view of the Scottish Government on how confidentiality in the negotiation of Patient Access Schemes can ensure best value for the NHS.
We recommend the Scottish Government provide detail of "...the proportion of Scottish Medicine Consortium submissions that include a PAS, what proportion are regarded as simple or complex and what proportion of complex PAS are accepted."i
Interaction with the pharmaceutical industry
In the Review of Access to New Medicines, Dr Brian Montgomery stated—
"To date the interaction between the various players, and certainly that between NHSScotland and the pharmaceutical industry has tended to begin only once a medicine has been granted a license and a submission to SMC is being considered. All spoken to consider this to be too late, missing as it does the opportunity to collaborate on issues such as horizon scanning, the optimal use of specific medicines, wider assessments of impact and value and more pragmatic pricing strategies."i
We also heard there was still a case for review of interaction with the pharmaceutical industry, with groups such as the European Medicines Groupii, Merck, Sharp and Dohmeii and Janssensii calling for further engagement with companies. The Blood Cancer Alliance suggested a "more flexible, better resourced and transparent commercial framework for negotiation with the industry is essential if the most clinically effective medicines are to be made cost effective in the future."ii
The British Dental Associationii called for stronger negotiations with drug companies in order to save money. The role of the SMC is not to negotiate on price, merely to assess whether the benefits of the medicine offered are worth the stated cost. This left us wondering where the room for negotiations on price are within the tendering and national procurement systems.
We recommend the Scottish Government and the Scottish Medicines Consortium consider the views on interactions with the pharmaceutical industry during assessment of new medicines we received and what benefits could accrue for the NHS in Scotland from improvement in this area.
Access to new medicines - conclusions
Although consideration of access to new medicines did not form part of the inquiry, we have considered views presented on the role of the SMC in as far as this impacts on the clinical and cost effectiveness of medicines used in the NHS. While we believe advances have been made in light of the recommendations of the Montgomery Review, we urge the Scottish Government to consider the views proposed to us on further potential improvements. We also suggest there is scope to consider the operation of Patient Access Schemes further to ensure these are best serving the NHS and to look at interactions with the pharmaceutical industry during assessment of medicines.
Scrutiny of non-medicine interventions
Throughout the inquiry, we heard a general theme there is inequality of scrutiny between medicines compared to other healthcare interventions.
Other interventions such as medical devices, appliances, technology, and procedures do not receive the same robust level of scrutiny as medicines before being used on patients. It is also not clear to us how the potential benefits of medicines are assessed against the benefits of non-medicine interventions.
On licensing, the Cabinet Secretary for Health and Sport, Jeane Freeman, told us "We continue to press the [Medicines & Healthcare products Regulatory Agency] MHRA to make its process for medical devices comparable with the process that we all expect for clinical trials, licensing and so on of medicines."i She suggested the Medicines and Medical Devices Bill at Westminster may be a vehicle for discussions on improving the MHRA's processes. We requestedii details of the discussions which have taken place on this issue with the MHRA and look forward to considering a legislative consent memorandum on the Bill.
Dr Ewan Bell, National Clinical Lead, Area Drug and Therapeutics Committee Collaborative, Healthcare Improvement Scotland, said—
"..there are other interventions that are non-medical but are not social prescribing, such as bariatric surgery, which is much more effective for the treatment of type 2 diabetes than any medicine. There has not been sufficient evaluation of different interventions outwith medicines in NHS Scotland......The health boards have a variety of inputs—from the Scottish Medicines Consortium, the Scottish health technologies group, clinical guidelines, patient support groups and targets—and there is no national, once-for-Scotland comparison or health technology-type evaluation of those different inputs. There is no comparison of all those different interventions, and no ranking—I do not want to use the word “prioritisation”—of what NHS Scotland believes that it is important to deliver on."i
This issue is further discussed at length in the NON-PHARMACEUTICAL INTERVENTIONS and SOCIAL PRESCRIBING sections of this report.
David Coulson, Assistant Director of Pharmacy, NHS Taysidei suggested the single national formulary (considered fully at the FORMULARY section of this report) may be a way to consider national governance for the prescription of non-medicine interventions.
In June 2018, the then Cabinet Secretary for Health and Sport, Shona Robison, told us—
"A policy team for medical devices within the Scottish Government is in the process of being set up which will consider, with stakeholders, the need for further policies and strategies on medical devices' use in Scotland."v
We ask the Scottish Government to provide detail as to how the assessment process for new medicines compares the benefits of medicines with non-medicine interventions (such as surgery, digital technology and lifestyle changes).
We ask the Scottish Government to provide an update on the work of the established policy team within the Scottish Government considering use of medical devices in Scotland, including remit, timescales for work, engagement activities with stakeholders to date, the outcomes of this work and plans, including timescales, for further scrutiny and governance in the area of medical devices.
Purchase and Procurement

The process of purchase and procurement was one of the four central pillars of our inquiry.
Several submissions, predominantly from the pharmaceutical industry, focused on how budgets were already well managed and predictable due to pricing schemes and the close scrutiny of the drugs budget. The Association of the British Pharmaceutical Industry (ABPI) observed the "...percentage cost of what we spend on medicines in 2020 is only 1 per cent more than what was spent on medicines in 1948."i They also note "...and yet we have made great advances in this area over the past 40 years."i
To put this in a different perspective, Spending on medicines constitutes 13.1% of the overall health budgetiii totalling £1.76bn.
The wealth of evidence on spending on medicines we received will be considered in the following sections of this report—
Achieving best value in procurement;
International pricing markets;
Wholesale Supply Chains;
Pricing - branded medicines;
Pricing - generic medicines;
Purchasing in secondary care; and
Purchasing in primary care.
A. Achieving best value in procurement
In the previous chapter of this report, we considered aspects of the Review of Access to New Medicines which said—
"During evidence gathering from stakeholders much was made of the need to take a more sophisticated approach to benefits that includes patient reported outcomes, wider societal benefits such as the ability to continue working or a reduction in care or support requirements. The term “overall budget impact” was used on several occasions and requires the application of sophisticated health economic modelling which takes account not just of medicine costs but of whole system value and impact and introduces the concept of multiple “currencies” not just financial cost. The integration of health and social care presents Scotland with a particular opportunity in this regard."i
That report's author, Dr Brian Montgomery, explainedii to us how the options in terms of new diseases and how to treat them were exceeding the increasing budget being made available in healthcare. He notedii savings may be achieved from disinvestment in a particular drug but these may not be monetary, but the "currency" of "capacity".
David Coulson, Assistant Director of Pharmacy, NHS Tayside, and Rose Marie Parr, Chief Pharmaceutical Officerii, also warned against considering medicines in terms of their cost alone, with the former telling us—
"We need to consider the whole-system cost of medicines, which involves the issues you mention, such as the phlebotomist’s time, lab time and the use of reagents, and not just the procurement cost. We need to take that considered approach to medicines to ensure that we are making the right choices. We must make decisions that are based on sound clinical evidence."ii
B. International pricing markets
We were interested in how the UK's decision to leave the European Union would impact on pricing in the UK and Scotland of medicines.
Warwick Smith, Director General, British Generic Manufacturers Association (BGMA) was supportive of the regulatory landscape in the UK, which he said allowed the market to thrive, emphasising the importance of market forces in maintaining attractive prices when compared with more interventionist regimes in the rest of Europe.i
While Elizabeth Woodeson, Director of Medicines and Pharmacy, Department of Health and Social Care, saidi the UK Government had been clear the price of medicines would not be an issue for discussion in future trade negotiations, there was concerni about potential regulatory or licensing provisions which could be part of trade negotiations with the United States of America. The Cabinet Secretary for Health and Sport, Jeane Freeman, said it was too early to tell whether the outcomes of trade negotiations conducted by the UK Government would impact on price preferring to focus on "standards of governance, patient safety, and regulations that we want to see replicated"i.
C. Wholesale Supply Chains
We heard how the supply chain of medicines impacts on their cost effectiveness.
NHS Ayrshire and Arrani and NHS Scotland Directors of Pharmacyi each stressed the challenges posed by complexities and the time taken for procurement of medicines in both primary and secondary care. Community pharmacists also outlined the least efficient areas of interaction with the supply chain from their perspective although they were at pains to note how they did not allow challenges to impact on the service provided to patients (this is further explored in the COMMUNITY PHARMACY PROCUREMENT section of this report).
Warwick Smith, Director General, British Generic Manufacturers Association (BGMA), toldiii us delays in the supply chain could arise from issues such as availability of raw materials and active pharmaceutical ingredients (APIs), price competition, regulatory change, increased cost of goods, tender processes or penalties for non-supply.
The main points raised with us in terms of the supply chain were related to shortages.
Shortages
Warwick Smith (BGMA) providedi us with examples of why shortages of medicines occur, such as manufacturing changes abroad and reduced capacity due to adherence to regulatory requirements for packaging. He also notedi how this could impact on individual ingredients and how safety concerns could contribute to shortages.
Martin Sawer, Executive Director, Healthcare Distribution Association, defended the current approach suggestingi the decision to leave the European Union had caused the NHS to become better at analysing shortages and horizon scanning, stating there was a "much better understanding of how much stock is around"i. He further described steps taken by wholesalers to improve efficiency within the system, such as updated communication systems to provide better intelligence to pharmacists as to when they may expect stock to be available. He also advocatedi working with the private sector who he said were incentivised to prevent or plug gaps, rather than establish centralised supply management.
Both Warwick Smithi and Martin Saweri noted ongoing European wide strategic discussions on resilience in the supply chain and the geographical centralisation of production of certain medicines and chemicals. We were also interested in the impact of leaving the European Union in terms of the current 'just in time' approach to drug supply and how potential border checks would affect this.
Similarly, Martin Sawer expressed concern about any potential barriers which could impact on price. Alison Culpan, Scotland Director, Association of the British Pharmaceutical Industry (ABPI), said the organisation wanted a "frictionless border so that our medicines can come straight in"i. Angela Timoney, Director of Pharmacy, NHS Lothiani, spoke of the impact of the 'just in time' approach and said where the normal rate of shortages was around 80, there were currently around 120-130 shortages. She praised the efforts of national procurement to mitigate shortages.
Despite assurances to the contrary from industry the number of shortages is steadily increasing and we recommend the Scottish Government discuss this with industry representatives as a matter of urgency.
D. Pricing - Branded Medicines
Voluntary Pricing and Access Scheme (VPAS)
Spending on branded medicines is partly controlled by the Voluntary Pricing and Access Scheme (VPAS) which "caps the rate of growth in spend on branded medicines at 2 per cent. Spend above that is reimbursed; payments are made by industry to the NHS in England—and in Scotland, through the arrangements that we have to repay the devolved Administrations."i It is "expected to save the NHS in Scotland around £90 million in 2019"i. Both the UK Governmenti and industryi were enthusiastic about collaboration and negotiation between them in establishing the scheme.
The advantages of the scheme with regard to spending and cost effectiveness for the Scottish NHS are—
Predictability of budgets - several stakeholders were keen to emphasise how the scheme ensured predictability by capping rises in spending at 2%.
Applies to a significant proportion of medicines - the ABPI noted "In 2018, 58% of medicines accepted for use by the [Scottish Medicines Consortium] SMC were discounted and of the top 300 branded products used in the NHS, 90% had a patient access scheme discount attached."v
Accelerates and streamlines access to medicines - Merck, Sharp and Dohme suggestedv this was particularly helpful in the context of the UK leaving the European Union and it suggested the pricing scheme was a "crucial element of securing global confidence in the UK as a market post-Brexit".
A good model - Roche Products Ltd saidv the principle of a pricing agreement should be replicated across other areas of NHS spending to ensure "stability and surety for the overall finances of the NHS in Scotland".
Allows for commercial flexibility - Kwoya Kirin International highlighted how this allowed for flexibility regarding pricing for different indications (the specific use for a medicine where there may be more than one - see the MULTI INDICATION PRICING section of this report for further details).
However, there were also notes of caution in accepting the virtues of VPAS. The Blood Cancer Alliance advocatedv managing budgets was not the same as delivering value, and this was determined by the SMC.
Efficacy of the Voluntary Pricing and Access Scheme (VPAS)
Elizabeth Woodeson, Director of Medicines and Pharmacy, Department of Health and Social Care, UK Government, told us the UK Government believed "VPAS is an effective mechanism for capping the rate of growth in branded medicine spend, which is, by far, the bulk of expenditure on medicines"i.
Transparency in pricing around the UK
The VPAS includes a clause stating—
"The details of national commercial arrangements agreed with the purchasing authority in one UK country will be made available on a confidential basis to purchasing authorities in any part of the UK. Scheme Members will work with purchasing authorities to achieve comparable arrangements that provide an acceptable value proposition in each part of the UK."i
Written submissions we received were supportive of the ability to share such information and Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government, notedii transparency of pricing had been a Scottish Government priority in the 2018 negotiations.
However, others noted there was still discrepancy in pricing around the UK, despite this clause. In its written submission, Cancer Research UKiii noted these existed despite the VPAS commitment which should drive value for money in future commercial negotiations with medicines manufacturers.
NHS National Services Scotland (NHS NSS) agreed, observing instances where the price offered in Scotland has been higher than the price offered in England, adding—
"This is directly linked to differences in health technology assessment (HTA) processes."iv
Elizabeth Woodeson said the aim of the scheme was to achieve transparency in pricing across the UK but that this "does not necessarily mean that each country is required to agree the same price."v
Alison Culpan, Scotland Director, ABPI, expanded on this by stating the organisation "encourages its members to give each of the four nations the same arrangement"v and described how other elements may impact on the price paid for a medicine in each country (such as the collection of data). Merck, Sharp and Dohmevii elaborated, comparing the lack of provision for innovative pricing provided through the Commercial Framework and the data collection requirements of the Cancer Drugs Fund in England. They said "The Scottish system requests equitable pricing but equitable pricing does not mean equitable access for Scottish patients. Scotland cannot expect equity on one parameter without providing it in another".vii Alison Strath, Principal Pharmaceutical Officer, Scottish Government, added "We need to consider the different parameters that are sometimes attached to deals."v
Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS NSSv, said provision to share pricing information among purchasing authorities was in the process of being implemented and her organisation was "delighted" to see "provision for comparable arrangements to be offered to other home countries" in the VPAS agreement. She added work was ongoing to implement the VPAS provision on ensuring comparable arrangements around the UK.
We were surprised to hear UK wide pricing information was not collected, seeing this as a potential source of savings by learning from the approach of comparable countries and sought to discover if the Scottish Government was monitoring whether counterparts received better prices for the same products and if so, on what basis this was achieved. Alison Strath, Principal Pharmaceutical Officer, told us the Scottish Government was "setting up arrangements that will allow information sharing between the organisations that are involved in pricing and negotiations on price, so that we have sight of the prices and can enter negotiations about them."v We were also surprised to hear from NHS National Services Scotlandxii board purchasing and stock holding data would assist in the secondary care procurement process, but this is not currently gathered.
The issue of how pricing around the UK was monitored and shared was at best unclear to us, meaning it is hard to see how Scotland can be comparing the price paid and the arrangements on which these are based to achieve best value.
We recommend the Scottish Government routinely collect information on pricing around the UK along with the arrangements on which those prices were agreed, with a view to maximising the opportunity to obtain the best price for medicines.
We recommend the Scottish Government ensure the NHS is gathering data on purchasing and stock holding by individual boards to improve procurement processes.
Rebate to Scotland and the New Medicines Fund
The ABPI noted that while spending on medicines in 2017/18 was stated at £1.8bni, the actual cost would be lower thanks to rebates and confidential discounts offered by the industry through VPAS.
The Scottish Government has chosen to allocate most of the funding received from the rebate to the New Medicines Fund which Rose Marie Parr, Chief Pharmaceutical Officer, described as "a really positive policy"ii. She continued—
"People can see the increase in medicines and the increase in medicines use, but they can also see that rebate being used transparently for the new medicines fund. Boards have gained over the past five years and they are due to gain up to £90 million or so in the current year."ii
She later noted "To date, around £200 million has been made available to Boards in Scotland through the New Medicines Fund which is a significant contribution to achieving value based pricing itself."iv However, representations from pharmaceutical companies and others highlighted the VPAS scheme and rebates returned £258m to the NHS in Scotland in the last five years. Bristol-Myers Squibbv also suggested the fund was not transparent and called for more data on how this was used by boards.
We were also interested in the extent to which the New Medicine Fund met boards' need with regards to spend on new medicines. David Coulson, Assistant Director of Pharmacyii, said affordability of new medicines was challenging and boards relied on the advice of the SMC as to whether a medicine was worth the resource. The Cabinet Secretary for Health and Sport, Jeane Freeman, said—
"My suspicion is that, in truth, the fund will never be big enough, because pharmaceutical companies will continue to introduce new medicines that people will want to access."ii
We are interested in what happens to applications for end of life, orphan and ultra-orphan medicines when the fund is exhausted. The Cabinet Secretary also suggested there was a need to consider how personalised medicines would be funded in the future.ii
"The new medicines fund is vital, but we need to parallel the work that the SMC is doing in considering its processes in anticipation of precision medicine and coming innovations. We need to consider how we fund and whether we should alter our approach in the light of innovative and important developments for patients."ii
We ask the Scottish Government to clarify the significant discrepancy in the level of rebate received and the amount allocated to the New Medicines Fund.
We request a detailed breakdown by Board of the spend on new medicines each year and the amount they have received from the New Medicines Fund.
Voluntary Pricing and Access Scheme - conclusions
While we welcome the principle of a cap on the cost paid by the NHS for branded medicines and the willingness of the both the UK Government and pharmaceutical companies in negotiations on this, it is unclear why the cost is simply not capped, rather than a system of overpayment and rebate. We are of the view this must lead at the very least to inefficiency and unnecessary spend in terms of time and administration.
Pricing based on other considerations
Value and outcome based pricing
Several submissions proposed pricing systems based on outcomes and value. Community Pharmacy Scotland proposedi a system of weightings to reflect the benefits of a medicine in pricing. Others described the various benefits of and values of an outcome based approach to pricing, including reducing investment risk for the NHS while rewarding the pharmaceutical industry for innovation and therapeutic breakthroughs.
Elizabeth Woodeson, Director of Medicines and Pharmacy, Department of the Health and Social Care, UK Government, detailedii the role of the National Institute for Health and Care Excellence (NICE) and the Scottish Medicines Consortium (SMC) in assessing new medicines and said they look at the benefit a medicine delivers as well as its price.
However, it was clear to us "benefit" was not the same as the outcomes from taking the medicine and Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government, notedii the potential differences between the reactions to medicines, and benefits, demonstrated during clinical trials and the "real-world"ii application.
Throughout our inquiry, we have consistently been told data on outcomes is not collected. We therefore asked for further information about outcome based pricing from the Scottish Government.
We were told—
"The Committee also asked for my view on what should happen if the outcome, once assessed, differ from the original claims by the company. As you will know, there is a greater interest from the pharmaceutical industry to explore outcomes-based pricing and commercial agreements and we are actively considering how the VPAS might provide new opportunities in relation to innovative and flexible approaches to pricing. Critical to the delivery of novel pricing approaches will be the NHS capability to collect outcome data, which is why our strategic approach to data collection and the interdependency with the Digital Health and Care Strategy is important. I would imagine that in the event that the outcomes are different there would be the opportunity to consider a number of options, including whether to reconsider the reimbursement price, review and/or its place in any prescribing guidance."
We were previously told the Scottish Government was testing a proof of concept outcome based pricing approachv and soughtvi details of other innovative and flexible approaches to pricing being considered in correspondence to the Cabinet Secretary.
Multi-indication pricing
The European Medicines Groupi, Roche Products Ltdi, ABPIi and Kwoya Kirin Internationali noted pricing for medicines could depend on the indication for which they were proposed to treat, with the latter saying—
"A single price cannot reflect all these values thus to provide all these options in a cost effective manner, some flexibility on pricing is essential".i
This would represent a savings potential by ensuring costs paid for use for one indication were not dictated by the potential higher costs which could be demanded for use to treat another indication.
Pricing based on other considerations - conclusions
We heard overwhelming support from a variety of stakeholders for innovations in pricing on value and outcomes, but this served to highlight the lack of collection and utilisation of this valuable information. Bristol-Myers Squibbi proposed a working group should be established to consider Managed Access Schemes, including innovative pricing.
We recommend the Scottish Government consider establishing a group to consider innovative pricing schemes in the round, notwithstanding the urgent need for outcome data collection to be considered across the system for various purposes.
E. Pricing - generic medicine
Audit Scotland describe generic medicine as "cheaper, sometimes significantly, compared to branded medicine"i and note cost saving measures implemented by boards included increased use of generic medicine in secondary care, reviews of prescriptions in primary care to allow for cheaper options to be used and use of biosimilars (which again may be cheaper). A recent report by Oxera demonstrates the potential savings to be made by prescribing generic version of drugs, noting—
"While there is variation in the extent and speed of price changes across different products, on average, the generic price in the six months after loss of exclusivity is 70% lower, falling to 80–90% lower over a four-year period."ii
The report also describes the UK as an attractive market for manufacturers of generic medicines due to "large size and low regulatory barriers"ii and analysis in the report uncovers the cost of generic medicines in the UK is generally lower than in other European countries. We also heard about fewer barriers to entry compared to other European countries.
Cost of generics - price increases
Oxera describes how market conditions can impact on the price of generic medicines—
"...manufacturers have the ability to change their production levels and prices relatively easily to react to changes in market conditions. In the short term, manufacturers generally avoid exiting entirely, instead decreasing production if market conditions are adverse and increasing production if market conditions improve. This creates a self-correcting mechanism whereby short-term and significant price increases are often met with additional supply followed by a decrease in price."i
The Royal Pharmaceutical Society in Scotland toldii us the prices of generic medicines represented good value for money, but that these were more susceptible to price fluctuations than other drugs. One of the reasons offered for this was shortages in the global supply chain and the sudden impact this could have on the cost of generic medicine. Janssenii and AstraZenecaii cited NHS figures showing the biggest price rises reported by ISD Scotlandv over the last two years had been for generic medicines, with the former suggesting this may be due to "paying more for items in short supply". The British Generic Manufacturers Association (BGMA)ii suggested a longer lead in time of 4 months in secondary care procurement could lessen the impact of shortages.
Martin Sawer, Executive Director, Healthcare Distribution Association, expressedvii concern about any potential barriers caused by border arrangements which could impact on price.
NHS Grampian Area Drug and Therapeutic Committee gave an example of the power and actions of drug companies in relation to price indicating—
"Value is also undermined when elements of the pharmaceutical industry hike prices of niche products with little competition. Often these products are older established medicines or medicines that have a significant use as unlicensed medicines from specialist suppliers. These medicines require little in the way of return on investment for research and development but are able to be priced high on the basis of little or no market competition. These practices can impact significantly on NHS spend with no improvement in patient outcomes."ii
Cost of generics - UK Government price setting powers
In 2017, the Health Service Medical Supplies (Costs) Act 2017 gave ministers the power to intervene to control the price of generics when there is insufficient competition. To date those powers have not been used. We queried this with Elizabeth Woodeson, Director of Medicines and Pharmacy, Department of Health and Social Care, UK Government, who toldi us the Government had simultaneously taken powers to require pharmaceutical companies to justify any price increases. She said it was hoped agreements on price could be reached with companies prior to requiring the price setting power and she described this move as a "significant intervention in a marketplace"i. She also noted despite the Government having legislated that work was being done to consider how the power could be used and a forthcoming consultation was expected. She also confirmediii the price setting powers, once exercised by a UK Secretary of State, would apply around the UK.
The BGMA, while welcoming the powers of the UK Government to investigate and act, saidi price rises for generic medicines were justified in some instances but they also suggestedi that where demand was low competitive pricing could not work. The BGMA suggestedvi streamlining of the MHRA approvals process would be preferable to "intervention in the market" as a means of improving access. Their overarching view being that it "is wrong to have different pricing and reimbursement mechanisms for unbranded and branded medicines"vi suggesting different mechanisms were required for "products that face competition and those that don't."vi
F. Purchasing in Secondary Care

There are separate procurement routes for medicines depending on whether these are prescribed in primary or secondary care. Roughly, the latter tend to be more expensive using more branded medicines to treat more serious ailments.
According to the Scottish Parliament Information Centre—
"Medicines expenditure in Scotland has risen steadily over the last decade and it now accounts for 13.1% of the total health budget. Most prescribing takes place in the community but recent trends show that prescribing costs in this sector have reduced. However, drugs expenditure in the community and hospitals has been growing at a higher rate than overall expenditure."i
Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS National Services Scotland (NHS NSS), informedii us the previous year had seen a slight "deflation" in the centrally procured medicine spend for secondary care. Of a £400m spend, £58m of savings elsewhere had been achieved and she demonstrated this with examples of procurement of hepatitis C drugs and use of biosimilars (which Dr Alan MacDonald, Chair of the Scottish Medicines Consortium also describedii as a "good news story").
Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clydeii told us medicines represented 17% of board expenditure in Scotland. She said a major challenge for controlling spend was increased access to medicines which had been developed as a result of the Montgomery Review. Both sheii and Angela Timoney, Director of Pharmacy, NHS Lothianii, provided examples of best practice where boards had been able to significantly reduce their overall spend on the medicines, but suggested there were likely to be incidences of use of branded or more expensive medicines in hospitals by the nature of their work.
NHS Ayrshire and Arran said "The increasing availability of very high cost medicines in acute care has resulted in a percentage rise in hospital spend that exceeds Board's annual uplift. A large part of offsetting these costs has to be met by targeting savings in other areas of care."vii
Community Pharmacy Scotland proposed that—
"products’ list prices in secondary care (which are set by manufacturers and sometimes known as ex-factory prices) do not always reflect the ....NHS net price because confidential discounts are agreed, including via patient access schemes. Consequently, spending measured by list prices could overstate the total cost of medicines in secondary care."vii
Roche Products Ltd also assertedvii the advertised list price of a drug did not always reflect what was ultimately paid by the NHS.
G. Purchasing in primary care

According to the Scottish Parliament Information Centre—
"The vast majority of medicines are dispensed to patients in the community using a prescription issued by a General Practitioner (GP) or another prescriber such as a dentists or nurse. These prescriptions are usually taken to a community pharmacy and dispensed by a pharmacist. Like GPs, community pharmacists are not employees of the NHS but instead they operate as independent businesses contracted by the NHS. There are currently 1257 pharmacies providing pharmaceutical care services in Scotland......Each community pharmacist procures medicines to fulfil prescriptions on behalf of their patients. This is generally done by procuring the required medicine from wholesalers who effectively act as a middleman between the pharmaceutical companies and pharmacists. Some manufacturers may offer a direct to pharmacy arrangement for the procurement of medicines (i.e. bypassing the wholesaler)."i
We were surprised to hear from Community Pharmacy Scotland they could see no improvements to the efficiency of procurement in primary care, as evidence presented to us suggested there are areas, not least in terms of pharmacist time, where they system could be streamlined. This is further explored in the following sections.
Community Pharmacy Procurement
In the main, those pharmacists and NHS National Services Scotland (NHS NSS), who all worked within the system were supportive of the existing processes of procurement of medicine in primary care. This is done via community pharmacy seeking to obtain the best prices from wholesalers.
However, challenges for community pharmacists were noted—
The efficiency of community pharmacy procurement; and
Movement of prescriptions traditionally administered in secondary care settings to primary care settings (the example cited most commonly was the ability for patients to take hepatitis C drugs in the community);
Martin Sawer, Executive Director, Healthcare Distribution Association, supported the current schemes, saying—
"We support the flexibility that has been created to enable generic prices to rise. It allows for a bit more resilience, because new manufacturers will enter the market, and it creates incentives to purchase in the supply chain. As wholesalers, we try to get the best deal from manufacturers because we own most of the products that we distribute. Likewise, pharmacies use wholesaler competition to try to buy and purchase in the most attractive way, based on the reimbursement prices that they will get. We certainly think that incentives in the supply chain provide a lot of competition across the UK, and that volume is important to keep supply going."i
Efficiency of Community Pharmacy Procurement
Stakeholders such as Community Pharmacy Scotlandi and the National Pharmacy Associationi were at pains to promote the efficiency of procurement through community pharmacies and, although they noted challenges, emphasised the lack of impact these had on the end result for the patient and the NHS.
While promoting their efficiency, they also took every opportunity to tell us of the time spent by senior pharmacists in procuring pharmacy stock. Evidence focused on—
The wholesale model and how this impacts on the efficiency of community pharmacy procurement; and
The seniority of staff involved in procurement within community pharmacies
Staff
Recognising the issue arising above in relation to the time pharmacists are dedicating to procurement at the expense of directly interacting with patients, we were keen to understand why this task was being undertaken by senior staff.
The Royal Pharmaceutical Society in Scotland (RPS)i noted there could be a greater role for pharmacy technicians, particularly in dispensing, but said a more formalised career path was required. The RPS also suggestedi the correct level of qualified personnel should be assigned to the correct tasks to ensure efficiency, best value and more patient facing time for pharmacists. When asked how they were progressing this, the RPS responded they had "advocated extensively for improvements in the career pathways of pharmacy staff"iii but noted, without offering proactive solutions themselves, there was still much to be done. Matt Barclay, of Community Pharmacy Scotland, said "Technicians and other pharmacy team members can generally deal with this although...the pharmacist should have oversight around the supply chain, training for pharmacy team members on how to source medicines and may have to step in at times to ensure supply or source alternatives for patients."iv Angela Timoney, Director of Pharmacy, NHS Lothian, toldv us of work undertaken within the board to maximise the skills of all levels of staff.
She alsovi said there was a need to ensure technicians were doing some of the tasks currently fulfilled by pharmacists, but also involving support workers and administration staff in that. Scottish Government Chief Pharmaceutical Officer, Rose Marie Parrvi, agreed there was a need to work flexibly across job roles in the future which would be a new way of working and we sought details of this from the Cabinet Secretary for Health and Sport in correspondenceviii.
We consider this to be one of many examples of evidence presented to us of inefficiencies and issues with spending on medicines in the Scottish NHS by senior leaders, with no corresponding proposal of how to address it or recognition of the role they could play. We are clear the issue of how pharmacy staff time is used in primary care is inefficient and needs to be addressed as a matter of urgency. In community pharmacy settings, we suggest this includes removing the role of sourcing the cheapest medicines from pharmacists who, to improve efficiency, ought to be spending time sharing clinical expertise with patients.
We recommend the procurement of medicines become part of the formalised training for pharmacy technicians, including how to manage other staff to assist. We seek details from the Scottish Government as to how it will work with pharmacy bodies, such as the Royal Pharmaceutical Society in Scotland and Community Pharmacy Scotland, to effect this in primary care settings.
We also seek detail from the Cabinet Secretary as to any action the Scottish Government can take to implement more flexible working and formalised career pathways for pharmacy staff as a matter of urgency, including if appropriate using the community pharmacy contract.
Community pharmacy procurement versus central procurement
NHS National Services Scotland (NHS NSS) described the current system—
"The NHS reimburses pharmacies for medicines dispensed at a price higher than the cost to the pharmacy of purchasing the product...this is by design and a key reason for the effectiveness of the current arrangements. Community pharmacies retain the difference between the pharmacy purchase price for a medicine and the fixed Drug Tariff reimbursement price; this incentivises pharmacies to source medicines at the lowest available prices which creates the price competition that drives down prices being charged by wholesalers and manufacturers."i
If medicines cost more than the tariff price, pharmacists are also able to claim the surplus.
We were interested in whether it was more cost effective for the NHS to be reimbursing community pharmacists for medicines purchased than to be purchasing those centrally at the lower price offered by the drug company. The element of competition introduced by community pharmacy purchasing in a free market was key, according to NHS NSS.
"The current arrangements in place in Scotland and central procurement are both ‘free-market’ approaches that create a pro-competitive environment for generic medicines. The focus on competition through both methods is believed to be why the recent Oxera report into generic medicines prices found that UK prices were closer to countries in the study that made use of centralised tendering than those countries that applied regulatory approaches to control pricing."
Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS NSS saidii she believed the current system worked well and it can "achieve the same results as central tendering could achieve". She also informed us of comparative countries in Europe where central tendering took place and cited recent studies which suggested—
"...moving to a single central purchaser could lead to concentration of supply; risks associated with reducing the number of suppliers in a market include increasing the risk of supply problems and reducing price competition. These are both factors which can lead to higher prices over the long-term. Tender conditions (e.g. number of suppliers; length of award etc.) would need to be designed to manage this risk with the desire for low prices balanced with the need to ensure continuity of supply of medicines. A key benefit of the established approach in primary care is that it encourages plurality of supply."i
She also highlighted examples of where central procurement would or had been be preferable, such as for flu vaccinesii and off patent 'speciality' generics such as those for treating cancer and HIVi. She said the benefit of this would be allowing such drugs to be dispensed in community settings by bringing prices in line with those achieved in secondary care procurement.
The Scottish Drugs Tariff
The Scottish Drug Tariff stipulates the price a community pharmacist will be reimbursed for a drug and this is a set price regardless of what is paid for the medicine by the pharmacist. It is—
"...published for and on behalf of the Primary and Community Care Directorate, Scottish Government. The Tariff contains information regarding the prescribing, dispensing and reimbursement of medicines and appliances on primary care NHS prescriptions."i
Community Pharmacy Scotland noted "The method of determination of prices is according to a general protocol established by the Scottish Ministers after consultation with Community Pharmacy Scotland."ii
They providedii a detailed description of the operation of the tariff, including their view it is designed to encourage competition in the generics market, the nature of which meant it was robust and working well in the main.
Community Pharmacy Scotland added—
"Maintaining the delicate balance of the Drug Tariff supports existing competition and the positive impact of this competition on NHS pricing. Squeezing tariff prices too far could lead to generic manufacturers exiting the UK marketplace. The Tariff can occasionally be subverted at a local level through the practice of ‘branded generic’ prescribing. This is instigated by Health Boards looking for short term savings through certain companies offering cheaper ‘brands’ that the generic tariff price. This practice is fraught with challenges for patients, pharmacy and issues around sustainability of supply."ii
The Royal Pharmaceutical Society in Scotlandii said drugs should be included in the tariff in so far as possible to avoid fluctuations in price and to provide a continuity of supply.
The National Pharmacy Association said the Scottish Drug Tariff "has a more timely response process to medicines shortages and the resultant price increases than the rest of the UK, although it was recognised that on rare occasions contractors may still be out of pocket for dispensing prescriptions to patients."ii
Financial Risk
Matt Barclay of Community Pharmacy Scotland describedi the financial risks for community pharmacists of procuring more expensive treatments. He saidi the shift of delivery of treatments from primary to secondary care contributed to this. He notedi wholesalers payment terms were 30 days, but the NHS did not reimburse for 2-3 months, and described systems to apply for funding in advance as "clunky". Both hei and Lindsay McClure of NHS National Services Scotlandi referenced work underway on how the reimbursement system could remove the financial risk for community pharmacists.
We recommend the NHS pay community pharmacy on the same payment terms as have been requested by wholesalers.
Over the counter medicines
In the age of free prescriptions in Scotland, there has been controversy over the prescription of drugs like paracetamol and ibuprofen which can be purchased either over the counter or in other settings such as supermarkets.
We found consensus around the issue from many witnesses.
Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Governmenti suggested prescriptions for such drugs were often to manage long-term chronic conditions and it would not be cost effective or time efficient for a patient to purchase these over the counter as the amount they would be supplied would be very high. She also saidi the availability of drugs through the tariff was less important than how use of those drugs was reviewed, and suggested denying someone a prescription for a drug like paracetamol could require them to take something more harmful or more expensivei.
Dr Scott Jamieson of the Royal College of GPsi suggested spending on drugs available over the counter had reduced in his area and proposed this could be due to "realistic value conversations...along with polypharmacy reviews and the support of our wider multidisciplinary teams". He was at a loss to explain why prescribing of paracetamol had reduced but suggested it could be concerned with health literacy or quantity.
Purchasing and procurement - conclusions
We started by considering—
"...the challenge is how to best manage the interface between NHSScotland and the pharmaceutical industry. A situation where NHSScotland seeks to optimise its use of a finite resource subject to ever-increasing demands while the pharmaceutical industry operates in a competitive commercial environment and seeks to maximise return on its investment and meet the expectations of its shareholders."i
While agreeing with the sentiment of this, we believe a vital component has been omitted - the patient. We questioned whether it is in their best interest to allow market economics to determine the purchase and procurement of medicines.
We heard of the complexities of the international supply chain and the subsequent impact on the NHS, including the effect of leaving the European Union and the issue of shortages. We also considered the pricing of both branded and generic drugs.
Those involved in the industry were keen to emphasise the helpful nature of competition to pricing, but we were surprised to hear this echoed by those responsible for purchasing in the NHS, especially where it appeared to mean higher prices paid through procurement via the Scottish Drugs Tariff and community pharmacies.
We were pleased to hear of the successes of the Voluntary Price Access Scheme (VPAS) but lament the lack of information gathering on pricing around the UK to ensure Scotland is performing well in negotiations. We also question why the system of rebates is used, rather than simply restricting the amount which can be paid in the first place as this must lead to leakage. In our view, the New Medicines Fund is a welcome way to spend the rebates, however are not clear as to how boards fund access to new medicines once the allocation from the fund is depleted. The Scottish Government should ensure boards have sufficient, and if required ringfenced, funding to cover this. There also appeared to be a discrepancy in the rebate figure quoted and the amount being allocated to the fund.
Much of the evidence we received proposed innovative pricing schemes which would help reduce the cost and risk associated with medicines procurement in Scotland. Some proposals would also lead to better health outcomes for patients. However, the lack of information about the outcomes of use of medicines is preventing the introduction of such pricing mechanisms and as we discuss in the DATA AND IT section of our report, the time to comprehensively and uniformly update and improve data and technology use in the NHS is long since past.
Evidence received from pharmacists served to highlight the inefficiency of senior staff procuring medicines in primary care and we have suggested this be addressed as a matter of urgency. We have also proposed the NHS should pay suppliers on the terms they are subjected to.
However, our main concern is the difference in procurement systems for primary and secondary care and we don’t understand why one organisation, the NHS, has two concurrent methods operating. We question whether assessment has taken place to compare the prices achieved for the NHS via the two methods.
We recommend the Scottish Government assess the costs of procurement of all drugs purchased for use in Scotland via both primary and secondary care based prescriptions and produce comparative figures showing the costs of equivalent products purchased through the different routes.
We recommend the Scottish Government keep the Committee up to date with progress of discussions with the UK Government on Brexit negotiations and trade, as well as the outcomes of the negotiations with the EU, in the context of the likely impact on the cost of drugs in Scotland.
Prescribing
The next stage in the supply and demand for medicines is for NHS boards to decide which medicines they will make available to patients in their area.
The first step is the assessment by local Area Drug and Therapeutic Committees (ADTCs) for inclusion in local formularies. Prescribers then create prescriptions based on the medicines available on the formulary, among various other factors.
This chapter of the report will consider the various factors involved in the prescriptions of medicines and their influence on the clinical and cost effectiveness of treatments—
The freedom of the prescriber, including the main influences on prescribing behaviour;
Prescriptions in primary care;
Prescriptions in secondary care;
Non-medicine prescribing;
Social prescribing;
Realistic medicine;
Prescribing generics and biosimilars;
Prescription charges;
Prescribing non-licensed medicines;
Polypharmacy; and
Non-medical prescribers.
Freedom of the prescriber

The freedom of a prescriber in Scotland to prescribe what they consider appropriate was stated as one of the biggest influences on cost and clinical effectiveness in the system of supply and demand for medicines. Both the private and public sector submissions made similar points around this.
Factors which may influence prescribing
Audit Scotland described the main factors which can influence prescribing in its 2013 report "Prescribing in general practice in Scotland"i. It suggested these included—
The Quality and Outcomes Framework (QOF) (now replaced in the General Medical Services (GMS) Contract);
NHS Board formularies; and
Clinical guidelines.
A. The Quality and Outcomes Framework and the General Medical Services Contract (GMS Contract)
Audit Scotland described the Quality and Outcomes Framework (QOF) as—
"...a voluntary incentive scheme for GPs which uses financial incentives to encourage high quality care. The QOF has had considerable influence on the way GPs work, including their prescribing. For example, it includes targets for managing particular conditions, such as hypertension, which have an effect on prescribing."i
NHS Grampian Aii suggested QOF criteria on prescribing behaviour were not replicated in the new GMS contract and it is not clear what has replaced this mechanism. NHS Taysideii said in the absence of the QOF, the role of Health and Social Care Partnerships "to support and drive the sense of urgency regarding medicines spend" was vital in good medicine management.
We have further commented on the monitoring and accountability of the GMS contract in the GENERAL MEDICAL SERVICES (GMS) CONTRACT section of this report.
B. Formulary
A formulary is a list of medicines and medical devices which are available to prescribe. In his review of access to medicines, Dr Brian Montgomery said—
"Formularies perform two primary functions. They can be a comprehensive compendium of all medicines available for use or they can be decision-support tools which promote and support the safe and effective use of medicines in the management of medical conditions."i
Every health board has an ADTC which fulfils a number of functions with regard to the formulary—
Generate local formularies;
Provide an education function;
Take responsibility for prescribing decisions; and
Create local guidanceii and governance of medicines.
Once approved by the Scottish Medicines Consortium (SMC), local ADTCs will consider whether to add drugs to local formularies. As NHS Lothian ADTCii told us, not all drugs will automatically be added if there is already a range of choice on the formulary.
A number of issues were raised with us in relation to formularies—
Adding items to local formularies;
The value of a formulary with fewer options;
Formulary compliance;
Disinvestment/investment;
Competition; and
A Single National Formulary (SNF).
C. Clinical Guidelines
According to the Scottish Parliament Information Centre—
"Professional guidance to clinicians on prescribing states that they must 'provide effective treatments based on the best available evidence'. Doctors are expected to work within the limits of their competence and keep their knowledge and skills up to date. One of the ways in which they do this, is by using clinical guidance. There are a number of sources of clinical guidance, including professional bodies such as the Royal Colleges, the Scottish Intercollegiate Guidelines Network (SIGN) and the National Institute for health and Care Excellence (NICE)."i
Freedom of the prescriber - conclusions
We are of the view the prescriber in Scotland does indeed wield immense power when it comes to making clinically and cost effective decisions as to which medicines to prescribe and opportunities to improve their prescribing should be maximised.
The most prevalent current opportunity to influence behaviour presented to us in evidence was formularies. We concur these should be under constant review and Boards should take strategic decisions to disinvest where medicines are no longer the most clinically or cost effective options. We agree governance of non-medicine prescribing should be strengthened.
The biggest opportunity to influence behaviour which we believe is currently being missed is the use of the GMS contract to reward or incentivise prescribing of medicines which represent the best value for the patient.
Prescribing in primary care
Medicines prescribed in the community far outweigh those prescribed in hospital settings and the costs of this are shown below.
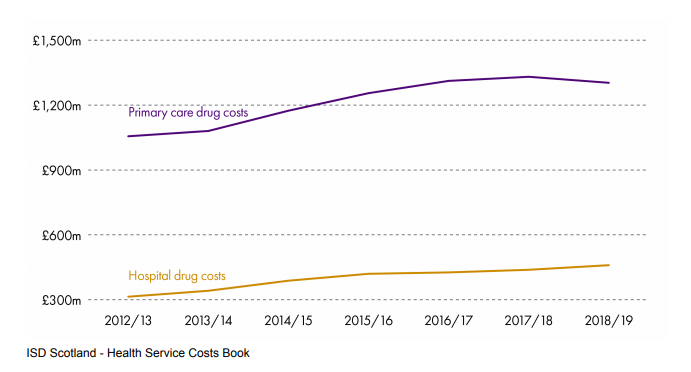
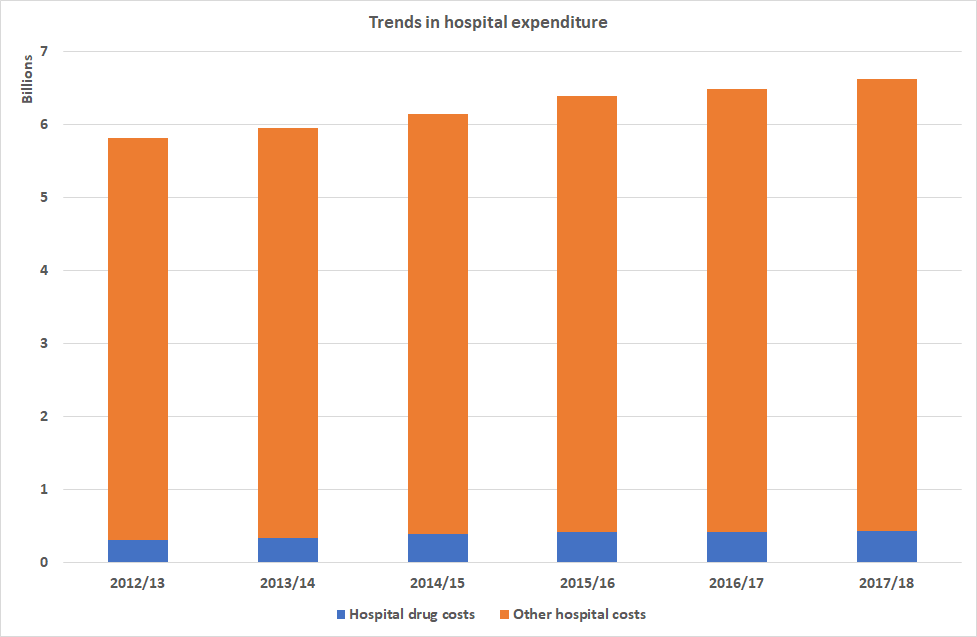
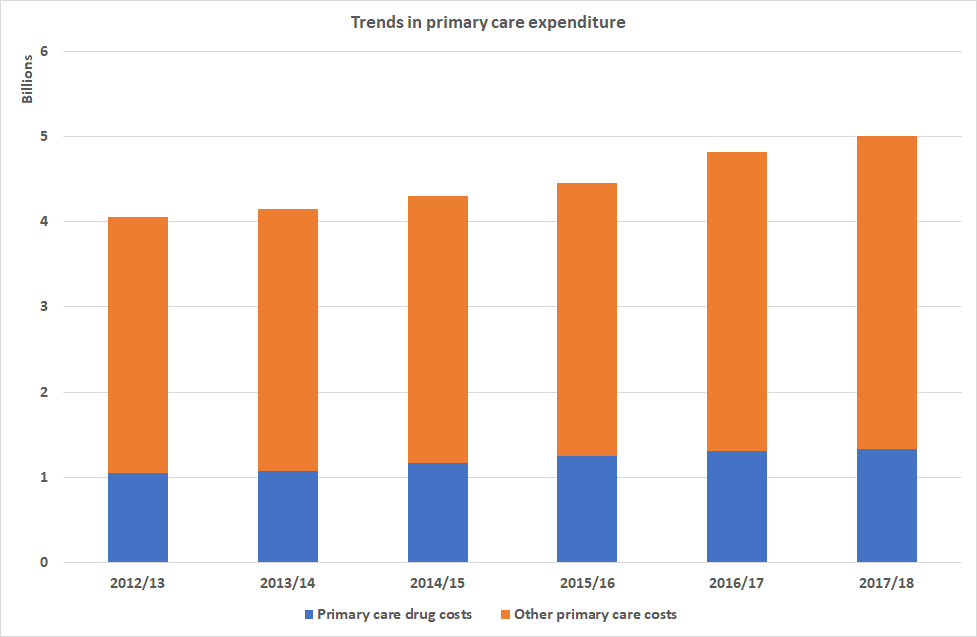
According to the Scottish Parliament Information Centre—
"The number of items issued in the community has steadily increased over the past decade, from 89.3 million items in 2009/10 to 103.4 million items in 2018/19 (+15.8%). However, the numbers have plateaued since 2016."i
It was also noted in "2018/19, the total cost of providing medicines and pharmacy services in the community decreased by 0.5%."i Audit Scotlandiii suggested this drop was due to better reviews of prescriptions, increasing the use of generic medicines in secondary care and switching to biosimilars.
Reviews of prescriptions
The issue of whether repeat or long-term prescriptions were being reviewed was prevalent throughout our inquiry. Several reasons for the importance of reviews were given—
Improved safety by addressing any misunderstandings about medicationi;
Provide an opportunity to discuss medicines with patients;
Reduce waste by avoiding patients remaining on medicines which are no longer effective for them; and
Prevent harm from taking medicines which are no longer suitable.
Audit Scotland said the Scottish Government Effective Prescribing team had helped boards reduce costs on medicines by "emphasising the importance of carrying out medicines reviews, to safely reduce the number of medications being taken at the same time."ii
However, from the evidence presented to us, it appears these are not happening comprehensively or systematically. NHS Tayside told us " Our current systems for review and discontinuation of medication fail to make significant inroads into those patients who are prescribed potentially inappropriate medicines."i
Comprehensive reviews of prescriptions are not routinely taking place
Several reasons were outlined to us as to why reviews may not be taking place—
A reluctance to change medicines, both on the part of the patient and the prescriber; and
Resources required to undertake reviews, including GP time and the pharmacotherapy aspects of the GMS contract.
In addition, we were interested in how the General Medical Services contract covers reviews of medication.
Patients' Views
The need for open conversations with patients on their medicines was emphasised throughout the inquiry, including on whether or not to change their prescriptions and prescription regimes.
Dr Scott Jamieson of the Royal College of GPs in Scotland spokei of the balance required between national decisions and individual patient preferences.
Other forms of support as part of reviews
Adam Stachura, Head of Policy and Communications, Age Scotland,i spoke of how reviews should not just be about medicines but should encompass other forms of care which may represent savings and better clinical outcomes for patients using the example of good podiatry services to enable better mobility.
Digitisation of reviews of medicines
The University of Strathclydei proposed using technology to ensure reviews of medicines were maximised, suggesting an app which could monitor adherence, patient response to medicines and the optimal time for switching to a cheaper alternative.
NHS Taysideii suggested a solution was to ensure all NHS boards were using ScriptSwitchii, prescribing software designed to suggest cost saving alternatives, and the Pharmacist and Data-Driven Quality Improvement in Primary Care (P-DQIP) interventionii, which aims to identify patients at risk from harm from polypharmacy and provide decisions support making functions.
Reviews of prescriptions - conclusions
The Chief Pharmaceutical Officer, Rose Marie Parr, told us people may be taking medicines from which they are no longer deriving benefit or may be now causing harm, and "better medication review systems as well as the capacity to review"i were required.
We agree with this and wonder what the Scottish Government, pharmacists and the NHS are doing to ensure this happens. Such reviews should also be used to encompass other forms of care which may represent savings and better clinical outcomes for patients, using the example of good podiatry services to enable better mobility.
The need for open conversations with patients on their medicines was emphasised to us throughout the inquiry, including on whether or not to change their prescriptions and prescription regimes. While we recognise there are challenges involved, none seem insurmountable in comparison to the prize to be won of better clinical outcomes for patients and cost effective use of medicines. It has been frustrating to hear of ambition in this area without associated action planning and of another area where GPs act unchecked.
Repeat prescribing
The management of repeat prescribing was an area of interest to us due to the obvious potential for waste to occur.
Medicines Care Review Service
One of the main solutions cited was the Medicines Care Review (MCR) servicei offered by community pharmacies. This is a three stage process, whereby patients with long-term conditions can register with their local pharmacy and undergo an assessment by the pharmacist to create a pharmaceutical care plan. The patient's GP then produces a repeat prescription for a period of time, which the pharmacist fulfils. Regular updates are sent to the GP after each episode of dispensing and at the end of the prescription period, a full report is submitted back to the GP.
Gail Caldwell, Director of Pharmacy at NHS Greater Glasgow and Clydeii, told us this was an incredibly important tool for boards to avoid waste in repeat prescribing. She said the system—
"...does not have the same risks as managed repeats, because each time the patient presents for a prescription, the pharmacist checks that the patient requires the medicine, which reduces the number of times that a medicine is dispensed. As part of that service, in order that we support patients to get the most from their medicines, patients get a medicines review. That is important, because it means that any inappropriate polypharmacy can be assessed and changes can be made."ii
Community Pharmacy Scotland suggested GP practices should engage with the MCRi and the repeat prescribing system could reduce the workload for doctors. We were also toldii the MCR service provided a "feedback loop" from community pharmacy to prescriber but this did not have a high uptake in the majority of areas. Technical issues with operation were also notedvi . Their ambitionii was for the service to provide a care summary akin to the patient treatment summary provided upon discharge from hospital. The benefit of this was cited as giving patients control over their own information and care.
Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotlandii spoke of the pharmacist as a "spotter" who could identify irregularities with repeat prescriptions and emphasised the importance of conversations with patients and passing on that information to pharmacists and GPs working within practices. Gail Caldwellii also spoke of the inbuilt design to encourage discussion with patients.
Again however, we were concerned by the lack of formality and structure surrounding the sharing of information as a result of these conversations.
Pharmacotherapy and repeat prescriptions
While Community Pharmacy Scotland and the Company Chemists Associationi suggested pharmacists within GP practices could assist with repeat prescribing, Jonathan Burtonii warned dealing with the administration of this exclusively would not make the best use of pharmacists' skills.
Delivery of repeat prescriptions
Jonathan Burton of the Royal Pharmaceutical Society in Scotlandi said patient involvement in delivery of repeat prescriptions was key to ensuring they were still getting medicines they were expecting and needing, although he did not elaborate on how this came about and our experience is of no patient input being sought.
Gail Caldwell of NHS Greater Glasgow and Clydei noted medicines could also be delivered to people's homes through the homecare system, which was how some manufacturers preferred their medicines to be supplied.
Campbell Shimmins of Community Pharmacy Scotlandi also extolled the benefits of having pharmacists in communities saying this meant they knew their patients and said changes to arrangements for getting medicines would result in direct contact with the patient.
We recommend the pharmacist role in repeat prescriptions be examined and, if required, included in the community pharmacy contract to ensure they are demonstrating value from the service provided and going beyond packaging and delivering medicines.
Prescribing in secondary care
The main themes of the evidence presented on prescribing in secondary care settings were—
Delaying discharge; and
The collection of prescribing data in secondary care and the Hospital Electronic Prescribing and Administration (HEPMA) System.
Delaying discharge
The issue of delayed discharge caused by the time taken to fulfil prescriptions for people leaving hospital arose.
Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clydei, and Angela Timoney, Director of Pharmacy, NHS Lothianii, said delays could be caused by—
time taken to create the prescription;
Accuracy checking; and
For the collaboration required between staff from various disciplines.
Gail Caldwell caused some Members of the Committee concern by indicatingi it takes 3 hours from receipt of a prescription to prepare it in hospitals and said there was a need to keep challenges posed by this, and the systems to mitigate it, under review as it was not person centred. When questioned as to why it takes so long, she indicatediv the doctor responsible for discharging patients would have to complete an Immediate Discharge Letter (IDL). However, we note this is concerned with the prescription and not dispensing of the drugs, and does not explain the time taken from receipt of the prescription in a pharmacy to leaving hospital. We were disappointed a Director of Pharmacy did not present plans seeking to eradicate such delays.
She suggested one solution to this could be transferring the prescription to a community pharmacist to fulfil, a proposition supported by Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotlandi. Graeme Bryson, Director of Pharmacy, NHS Dumfries and Gallowayi provided an example of a pilot being scoped in Dumfries and Galloway to use an electronic immediate discharge letter to communicate with community pharmacy.
Angela Timoney, Director of Pharmacy, NHS Lothiani, thought HEPMA would assist (further detail on the implementation of HEPMA can be found in the HOSPITAL ELECTRONIC PRESCRIBING AND ADMINISTRATION SYSTEM section of this report) and also highlightedii measures taken to ensure there was a full staffing compliment, noting shortages in the pharmacy profession as well as different qualification levels for different posts. She proposed pharmacists should be on the Shortages Occupation List (SOL).
We consider the time indicated for fulfilment of a prescription within hospital settings when compared with community counterparts is excessive and suggests inefficiency in the system. We are concerned this may be replicated across Scotland in other health board areas. We note the solutions to this being trialled in NHS Dumfries and Galloway and propose the outcomes of this may result in good practice which should be shared.
We ask the Scottish Government to assess how long prescriptions are taking in hospital settings in each health board across the country and the reasons for this.
We recommend the Scottish Government ensures the results of the trials taking place within NHS Dumfries and Galloway are shared among health boards as a starting point for addressing this inefficiency across the country.
Collection of prescribing data in secondary care
In general, we heard the ability and practice of capturing prescribing data in primary care works well thanks to e-prescribing, but this was not mirrored in secondary care. Evidence from various stakeholders, including Dr David Coulson, Assistant Director of Pharmacy at NHS Taysidei and Dr Scott Jamieson, Royal College of GPsi praised data collection in primary care, with a notable caveat from the British Medical Association in Scotlandiii suggesting electronic prescribing in primary care would improve data capture.
In secondary care however, the position is quite different.
Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government, told us—
"We can quite easily track the amount of drugs that are prescribed and who they are prescribed to on an individual patient basis. However, we do not have as good ways of tracking data in hospital and secondary care. We need to get prescribing decisions that are based on good data and good evidence and, in the longer term, we also need to think about outcomes or health gain in relation to those medicines.i"
The Chief Pharmaceutical Officer was optimistic regarding progress in collecting data, notingi good examples in relation to cancer, and when discussingi the roll out of the Hospital Electronic Prescribing and Administration system (HEPMA).
Hospital Electronic Prescribing and Administration System (HEPMA)
Throughout this inquiry we have been interested in the implementation of the Hospital Electronic Prescribing and Administration System (HEPMA).
The benefits of a system which collects and shares data on prescribing are clear. Witnesses and written submissions detailed the advantages and efficiencies to be gained from implementation, including—
Allowing hospital discharge letters to be pre-populated with prescription informationi;
Supporting ease of medicine reconciliationi;
"Improving the quality of prescribing"i;
"Reducing medicine information errors between primary and secondary care"i;
"Reducing missed doses of medicines"i;
"...better insight into prescribing and other medicine related activities through data analytics at different levels"i;
Removing the need for manual audits of prescriptions of medicines which are resource intensive for staffvii;
Allowing for comparison of data across all hospitals in Scotlandvii;
Facilitating analysis of variation in prescribingvii;
Increasing accountability for spending of prescribers and medicine management teamsvii;
Increasing prescribing from the formularyxi;
Improving safety by removing written prescriptionsxi;
Introducing electronic reminders as found in primary care into secondary carexi;
Facilitating the end of faxing of prescriptionsxi;
Mitigating delays in discharge caused by waiting for prescriptions to be dispensed in hospitalsvii;
Means only available medicines can be dispensed, reducing administration errors and delaysvii;
Introducing small time efficiencies for nurses which add up to more time with patientsvii; and
Requires all information to be included at the point of prescription, saving staff time in not having to go back and check detail with the prescribervii.
Despite all the advantages, Dr Lewis Morrison, Chair of BMA Scotlandvii, focused on the impact on the prescriber when he questioned whether the system would take longer to create a prescription than writing one by hand, although he acknowledgedvii electronic prescribing was safer.
Set against that concern, the Directors of Pharmacy for both NHS Lothianvii and NHS Greater Glasgow and Clydevii, were clear HEPMA would deliver efficiency into the system by removing the need to hand write documents.
In August 2017, the Scottish Government published "Achieving excellence in pharmaceutical care: a strategy for Scotland"i which included a recognition of the need to transform delivery of services through use of technology. In relation to HEPMA it—
"...committed to... implementation of Hospital Electronic Prescribing and Medicines Administration (HEPMA) in every NHS Board across Scotland.i"
In August 2017, three health boards had implemented HEPMA. We sought an update on progressxxv in December 2019, and in January 2020 were told—
"...NHS Lanarkshire is due to complete implementation in 2020. During 2019, NHS Lothian, NHS GGC and the North Region Consortium (NHS Grampian, NHS Highland, NHS Orkney, NHS Shetland, NHS Tayside and NHS Western Isles) all commenced their implementation, with the remaining Health Boards due to start in 2020-21. We will continue to work with Health Boards to ensure a local and regional approach to delivery across all the remaining Boards over the next three to five years and to support this we have established a national HEPMA Implementation Oversight Board."xxvi
We are concerned no new health boards have fully implemented the system in the 2 year period since the Achieving Excellence strategy document was published with no new health boards using the fully integrated system. That some boards have not yet started, and a 5 year timescale to implement a system commissioned in other areas, appears excessive.
Progress is disappointing and does not reflect the assertion the strategy for data collection "includes gaining traction and pace in terms of the implementation of Hospital Electronic Prescribing and Medicines Administration (HEPMA) across Scotland"xxvi.
In evidence, the Chief Pharmaceutical Officer said the Scottish Government is at "start of that journey" with regard to data collection in secondary care.
An update on implementation of HEPMA was committed tovii in the first quarter of 2020 and we were promisedxxix a copy of this report when available. This remains outstanding.
Non-pharmaceutical interventions
We received a wealth of evidence suggesting prescription of medicines was not always the most clinically or cost effective course of action, and that medicines should be seen only as part of a menu of options available.
The main points made to us were—
Assessment and governance of non-pharmaceutical interventions was not equal to that of medicines;
Medicines were not always the most clinically or cost effective course of action, with the example of diabetes cited on several occasions;
Meaningful discussions with patients are a vital part of the picture;
GPs and other prescribers need to have sufficient knowledge of non-pharmaceutical options in order to confidently suggest these to patients; and
Non-pharmaceutical interventions could be safer.
Assessment and governance of non-medicine prescribing
The assessment of some non-pharmaceutical interventions has been discussed in the SCRUTINY OF NON-PHARMACEUTICAL INTERVENTIONS section of this report. However, we cover it further here in the context of the improved governance called for. Stakeholders told us the assessment and scrutiny of non-pharmaceutical interventions was not of the same standard as that of medicines. This is despite the potential for similar or improved outcomes. Part of this relates to the standard of evidence prescribers seek of their effectiveness.
Dr Scott Jamieson of the Royal College of GPs toldi us the provision of non-medicines represented the biggest opportunity for review of governance and improvement in effective, and therefore cost effective, prescribing and told us of work undertaken in NHS Tayside to create good formulary processes for non-medicines underpinned by good review and governance structures.
We now understand the scrutiny of medicines is extremely robust in comparison to "other areas of NHS spending such as medical devices, procedures, IT infrastructure or contracted out services."ii Dr Jamieson suggestedi non-medicines are an area of "extremely high cost but without the regulatory supports that are in place for medicines"i. In addition, direct marketing to patients is permissible with non-medicines.
Dr Jamieson told us—
"chronic pain is a good example of an area in which there is pretty universal agreement that non-drug options should be more widely explored, because they are far safer to use and the evidence for them is as good as it is for medicine for chronic pain."i
Although adding a significant caveat, he said "Nonetheless, it is hard to get tangible evidence that is specific to a patient. With a multimorbid 85-year-old with five other diseases, for example, what are the caveats to the evidence that I need to present? It is really complicated and difficult to try to show that in an intelligent way that is accessible to me at the point of prescribing, and it is not yet being done."i
Dr Lewis Morrison, Chair of the British Medical Association (BMA) in Scotlandi, highlighted the difficulties in providing equal levels of evidence and suggested this may hinder, rather than help, the provision of non-pharmaceutical interventions, cautioningi that having to apply a similar standard of evidence might mean certain things never get done.
Medicines are not the only or even the most cost or clinically effective way forward
Many used diabetes as an example of where non-medicine forms of treatment may be the most effective. Dr Ewan Bell, National Clinical Lead, Area Drug and Therapeutics Committee Collaborative, Healthcare Improvement Scotland, proposedi bariatric surgery, the success rate of which he said could not be beaten by medicines in reducing diabetes, saying insufficient was being carried out in Scotland. He pointed to SIGN guidelines as being overly restrictive.
Dr Scott Jamieson, Royal College of GPs, saidi conversations as to the best course of treatment (be they surgical, lifestyle choices or medicines) should be taking place with patients, as per SIGN guidance, but noted misunderstanding of guidance could cause practitioners to reach for the prescription pad.
We are concerned SIGN guidelines could be misconstrued and recommend the Scottish Government work with SIGN to assess where ambiguity exists and update SIGN guidance to ensure full conversations with patients are not being hindered.
Several submissions discussed how the prescribing of medicines was wasteful where these are not the most clinical and/or cost effective treatment. Yet a number of witnesses described reasons why this was still happening.
The British Dental Associationiii said changes required to persuade the public to adopt lifestyle changes which would produce better health outcomes would require a level of Government intervention which may clash with other political priorities such as the economy. The organisation also suggested a monetary incentive for patients akin to that available for smoking cessation in pregnancy.
NHS Fife Area Drug and Therapeutic Committeeiii noted non-medical interventions may be the most clinically effective but assessment of this was difficult, adding access to the resources required were also challenging and limited. Dr Jamieson outlinedi work on decision making aids for patients, both internationally and within the Scottish Government. He spoke of the difficulties in finding evidence to support courses of action in specific cases and although he described information on conversations needed with patients as "binary and crude" he was supportive of Scottish Government action in this area. This topic is further discussed in the SOCIAL PRESCRIBING section of this report.
Several submissions noted the success of other lifestyle change policies such as smoking cessation and alcohol consumption and suggested these models should be looked at for other forms of healthy living which would improve health.
Dr Lewis Morrison, Chair of BMA Scotlandi and Eileen McKenna, Associate Director Professional Practice, Royal College of Nursing Scotlandi, both highlighted the role multidisciplinary teams have in keeping people well and in their homes, away from formal healthcare settings.
Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clydei saw a need for medicines to be part of a pathway of care alongside other interventions which may be more suitable, such as smoking cessation and flu vaccinations in the case of Chronic Obstructive Pulmonary Disease. She also spokei of the savings using more expensive anaesthetic drugs in some cases may have elsewhere in the system as part of a pathway of care.
Dr Jamieson suggestedx there was not currently sufficient time within a 10 minute appointment for GPs to consider non-medicine options comprehensively.
NHS Grampian Area Drug and Therapeutic Committee (ADTC)iii suggested that where medicines were used this should be complimented by appropriate lifestyle changes, but noted this required a different type of conversation with patients.
We understand there are challenges involved in prescribing the best course of treatment where this is not a medicine but are unwilling to accept this is a reason not to do it. Conversations with patients regarding treatment options do not appear to be taking place and appointment times are too restrictive. There is also no assessment or monitoring of ailments presented and treatments prescribed. We have considered these issues fully in the SOCIAL PRESCRIBING section of this report.
Communication with the public
Dr David Coulson, Assistant Director of Pharmacy, NHS Tayside was supportivei of campaigns around lifestyle changes and use of non-medicine interventions, and agreed community pharmacies would be a key player in dispersal of information.
GP knowledge
Dr Scott Jamieson of the Royal College of GPsi said GPs did not have the expertise to prescribe non-medical items, calling for further investment, and acknowledged as much time should be spent on such interventions with patients as medicines. He proposed the single national formulary model could help in this regard.
Other parts of the health service could be called upon to help in this regard. The Royal College of Occupational Therapistsii cited the results of a pilot in NHS Lanarkshire of occupational therapists (OT) working directly within GP practices and taking direct referrals. Working with an OT had produced a 55% reduction in the number of patients requiring further appointments with the GP with examples of where patients also had been removed from medicine regimes as a result of the changes.
Non-pharmaceutical interventions - conclusions
The area of non-medicine governance and prescription is one that needs to be further considered and there should be parity of scrutiny afforded to medicines and non-pharmaceutical interventions. We recognise the gaps in evidence but believe that all treatment decisions should be based on the best available evidence. We recommend this is an area for urgent strategic review, from procurement, through prescription, to consumption and request detail of how the Scottish Government intends to achieve parity.
Social prescribing
We have recently reportedi on our separate inquiry into social prescribing, and this subject has been prevalent during our work on medicines. A lot of submissions suggested a culture of instinctively reaching for a prescription pad while failing to explore alternative options, which could be safer for patients and represent cost savings for the NHS.
It was suggestedii to us not all GPs support the concept of social prescribing. Various reasons were cited including "time constraints, not perceiving it as part of their role, and a lack of strong evidence demonstrating the long-term effectiveness of social prescribing"i.
Culture change needed
Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government said "We can educate people to not think that a prescription is the first thing. We have to look at not just self-care but aspects of talking therapies and social prescribing. That involves a mindset change for patients and the public, and a discussion about that needs to happen."i
She and the Cabinet Secretary for Health and Sport, Jeane Freeman, both spoke on patient expectation with regard to prescribing medicines over other healthcare interventions. The Cabinet Secretary stated "We need to shift our mindset so that we see social prescribing as being at least of equal value to prescription of medicines."i
We are encouraged the Scottish Government is supportive in principle of the recommendation social prescriptions should be treated on an equal basis to medical prescriptions and we note the commitment in the Scottish Government responseiii to our report "Social Prescribing: Physical Activity is an Investment, Not a Cost"iv to explore how Scotland might achieve this aim.
We would welcome detail of the remit and timescales for this piece of work. We would also welcome further updates from Scottish Government as work progresses.
Evidence on the benefits and outcomes of social prescribing
In our report "Social Prescribing: Physical Activity is an Investment, Not a Cost"i we concluded—
"We are agreed and are clear that we do not require any further evidence in relation to the efficacy of physical activity on improving health and wellbeing. Direct correlation and causation has been proven and should be accepted by all sectors"i
The Cabinet Secretary for Health and Sport, Jeane Freeman, agreediii.
We were therefore disappointed to hear this view is not shared by general practitioners. Dr Scott Jamieson of the Royal College of GPs told us "We need to show evidence for them [social prescribing and non medicine options] that is as good as the evidence for the drug options, and we need to put as much investment into them so as to help our patients and to help our clinicians to make their decisions."iv
Dr Brian Montgomery callediv for data to be available on social prescribing to "convince us" the offering was "having the desired effect and it actually a worthwhile alternative". We profoundly disagree. We are of the view this evidence exists, and as we stated in our reporti, and agreed by Parliament, the time for collection of information about the benefits of sporting and cultural activities has passed and we have entered an era of focus on delivery.
An instinct to prescribe medicines
Dr Alan MacDonald, Chair of the Scottish Medicines Consortium (SMC), saidi SMC was "permissive" meaning an approval meant a medicine had been approved, not mandated, for use. He said prescribers should be aware of medicines available but were also free to choose other options. Dr Brian Montgomery expressedi the view the system was "skewed" towards the prescription of medicines and Dr David Coulson, Assistant Director of Pharmacy at NHS Tayside, suggestedi it was easy to follow the pathway of a prescription of a medicine in comparison to alternative options.
The Cabinet Secretary for Health and Sport, Jeane Freeman,i said there was a need to overcome the instinct to prescribe medicines, noting how the support of pharmacotherapy services and anticipatory care planning would contribute to this.
A pill for every ill - patient expectation
Several witnesses, including the Cabinet Secretary for Health and Sporti, spoke of the culture of a "pill for every ill" and suggested the patient expectation of being prescribed a pill was a hurdle to overcome in the path to full adoption of social prescribing and realistic medicine.
Dr Lewis Morrison, Chair of British Medical Association Scotland, i, said there were better levels of understanding and health literacy in Europe than here. He noted the risks of overloading patients with data when all they were interested in was outcomes and said patients "tend to focus on medication because it is quick". Adam Stachura, Head of Policy and Communications, Age Scotland i noted the pressure which could be applied on health professionals by patients to prescribe a pill, using the example of a complete change in diet to cure acid reflux versus taking a pill.
We were also interested in whether the community pharmacy contract created an incentive for them to dispense medicine rather than discuss alternatives as they will be recompensed for the drugs dispensed. Campbell Shimmins of Community Pharmacy Scotlandi suggested it may previously have been the case but the nature of the contract was changing to include more service delivery elements such as Pharmacy First.
The Cabinet Secretary for Health and Sport, Jeane Freeman, said "We need to help people to understand that even though they might not get those [medicines], they will still have been treated. We need to shift our mindset so that we see social prescribing as being at least of equal value to prescription of medicines."i
We welcome the Scottish Government's attitude to addressing the assumption of patients that medicines will be prescribed, but the evidence provided raised further questions. We are interested both in what the Scottish Government is doing to address the culture with the public but also how it is monitoring the conversations highlighted by Alpana Mair, Head of Effective Prescribing and Therapeutics, Scottish Government, involving the 7 Step Process are taking place (see the GENERAL MEDICAL SERVICES (GMS) CONTRACT section of this report for further detail). We sought the view of the Cabinet Secretary for Health and Sport on this issue in correspondencevi.
We would welcome detail of precisely how the Scottish Government proposes to change the culture and perception of the public that where the prescription of a medicine is expected during consultations and how it intends to support primary care providers to access social prescribing.
New General Medical Services (GMS) Contract and support from other health professionals
The new General Medical Services (GMS) contract introduces a new pharmacotherapy service, designed to integrate the work of GPs, pharmacists and other health professionals in one practice. This approach is described as "crucial to reducing GP workload"i and includes transferring responsibilities for work to pharmacistsii. The contract stipulates all GP practices will be serviced by pharmacy support by April 2021.
Dr Scott Jamieson of the Royal College of GPsiii and the British Medical Association (BMA)iv suggested the new GMS contract would increase the capacity for GPs to assess more complex patients by making better use of other primary care colleagues. He said this would allow for consideration of non-drug options and Matt Barclay of Community Pharmacy Scotlandv agreed other health professionals could support non-pharmaceutical prescriptions.
The Cabinet Secretary for Health and Sport, Jeane Freeman, said the new pharmacotherapy service represented an opportunity for progressing the social prescribing agenda, noting the conversations pharmacists have with patients and citing evidence people "find it easier to be honest with the pharmacist".v She suggested the pharmacist, in discussion with the GP, was best placed to introduce the concept of alternative prescribing.
This raises several questions for the Committee regarding the sequence of events involved and the circumstances under which a patient would speak to a pharmacist at a GP practice in advance of speaking to a GP. We are of the view this approach would rely on a carefully managed triage system within GP practices in order to ensure the patient spoke to the right healthcare professional within a practice and is therefore dependent on the relationship of trust between a patient and the individual ascertaining their needs.
We would welcome further detail from the Cabinet Secretary on precisely how the new pharmacotherapy aspect of the GMS contract will promote social prescribing and how this will be evaluated and monitored.
It also raises the question, once again, of what happens to the information patients provide to community pharmacists regarding their attitudes to medicines. We sought the view of the Cabinet Secretary for Health and Sport on this issue in correspondencevii.
The Cabinet Secretary also indicated the "opportunity to use community link workers"v. We previously welcomed the commitment to employ 250 community link workers throughout Scotland by 2021 and in the Cabinet Secretary's response to our report on social prescribingix, she said—
"The number of Community Links Workers in post as at March 2019, based on October returns, is 116."x
We ask the Scottish Government to provide detail of the number of community link workers currently employed and a progress update towards meeting the target of 250 by March 2021.
In the Scottish Government's responsex to our report "Social Prescribing: Physical Activity is an Investment, Not a Cost"ix it was indicated that Community Link Workers are allocated to practices based on an assessment of local need and in line with strategic priorities.
We would welcome an outline of how this supply and demand is determined. It would be helpful to see detail of any guidance on this for Integration Authorities.
Longer Appointment Times
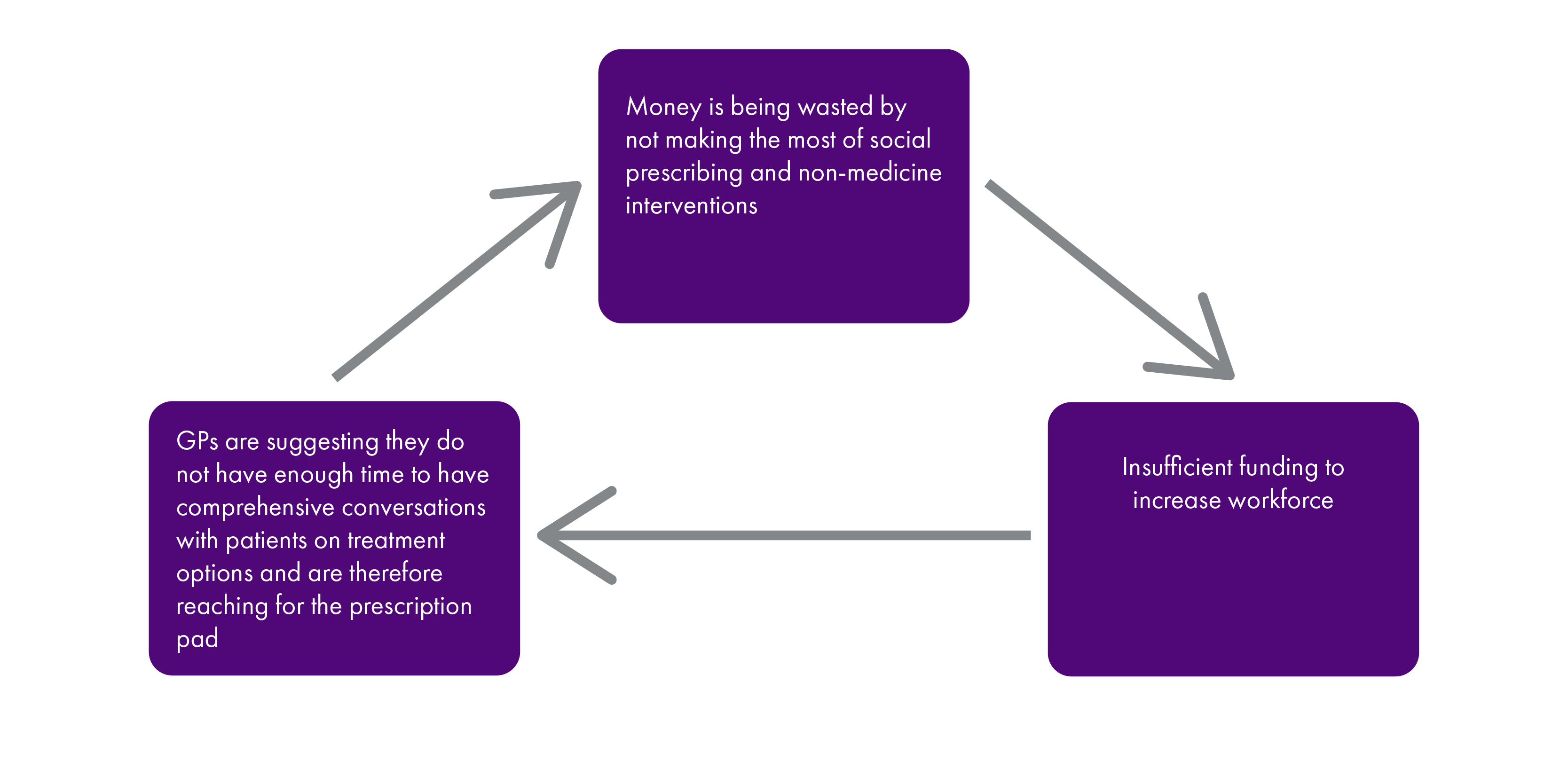
We recognise the pressures on GPs, but also note their self-employed status as business owners. It is entirely within their gift to ensure the social prescribing and realistic medicine agendas expected by the Scottish Government and mandated by the GMS contract, are delivered. Accompanying documents to the contract, such as the policy memorandumi to the regulations and the accessible versionii of the regulations, make clear holistic and comprehensive care for patients' body and mind is an underlying principle of the contract.
Eileen McKenna, Associate Director Professional Practice, Royal College of Nursing Scotland said "There is an issue with time: many professionals are time constrained. There is some evidence of nurse prescribers having longer appointment times, promoting non-medicine interventions, doing reviews and listening to individuals with regard to their compliance and whether they take their medicines. That is multifactorial: as you have heard, it is about multidisciplinary reviews, polypharmacy reviews, people having time and patients being empowered."iii
The Cabinet Secretary for Health and Sport, Jeane Freeman, said—
"....we need to find a way to actively promote social prescribing in a way that is practicable for the prescribers."iii
We once again return to the issue of evaluation of the contract and how the Scottish Government is ensuring GPs are meeting expectations set out in national strategies (as discussed in the REVIEWS OF PRESCRIPTIONS section of this report).
We ask the Scottish Government to provide its view on the appointment length of GPs and in light of the changes made in the GP contract, along with those for pharmacy, provide an assessment of the impact on GP time.
We reiterate our recommendation the Scottish Government provide full detail of how it intends to evaluate the GMS contract, including how GPs deliver policy priorities such as social prescribing and realistic medicine, and when the contract will next be reviewed to ensure it is maximising its power to encourage in prescribers the behaviours desired by the Scottish Government.
Awareness of referral pathways and activities to prescribe
We heard evidence on the lack of available referral pathways and awareness among prescribers of the options for activities to prescribe. Dr David Coulson, Assistant Director of Pharmacy at NHS Tayside, said "We are not good at making it easy to access other pathways, which do not involve medicines.i" and NHS Taysideii also called for further investment in public health initiatives to move from cure to prevention.
Dr Scott Jamieson of the Royal College of GPs suggestediii a lack of knowledge among GPs and lack of a formulary style system made it hard for doctors to socially prescribe and suggested this should be done by others with more awareness of the options. However, this suggests the onus would be on the patient to present their symptoms to the professional with expertise in the solution, inferring they would already have to know how to cure their ailment and thus rendering the need for medical intervention obsolete. We believe health professionals, and in particular GPs, need to have a better awareness of social prescribing.
Indeed, in our report "Social Prescribing: Physical Activity is an Investment, Not a Cost"iv we suggested there is a lack of comprehensive awareness of initiatives which can be prescribed. We made the following recommendations—
"We recommend Scottish Government supports NHS Boards and Integration Authorities (IAs) to invest in engagement work to raise awareness and understanding of social prescribing, and other primary prevention activities round promoting physical activity, and their benefits to the public.
We recommend IAs develop and roll-out awareness and education work of social prescribing and other primary prevention activities around promoting physical activity, across all heath and social care professionals."iv
Matt Barclay, Director of Operations at Community Pharmacy Scotland, said "Other practitioners in the multidisciplinary team can support that direction of travel by being aware of the options."i The Cabinet Secretary for Health and Sport, Jeane Freeman, said "We need to ensure that everyone in a general practice—the pharmacist, the GPs, the advanced nurse practitioners and practice nurses—knows about all the opportunities in the community that people can be pointed towards and helped to join; for example, book clubs, walking football groups, walking groups and lunch clubs."i
In the Cabinet Secretary's response to our report, she said this was being achieved—
The Scottish Government was "Supporting NHS Boards and Integration Authorities to implement physical activity social prescribing by embedding the National Physical Activity Pathway (NPAP) into existing practice."viii
NHS Scotland was working with academics to produce Quality Standards for Physical Activity Referral and NHS Scotland would "continue to raise awareness amongst health and social care professionals of the NPAP implementation guidance, e-learning opportunities such as Raising the Issue of Physical Activity, and resources such as Moving Medicine."viii
However, no specific detail was offered on how health boards and integration authorities are being supported to embed NPAP into existing practice, nor how awareness of physical activity prescription routes would be raised.
The Cabinet Secretary's response further described the NPAP process as an "evidence based pathway through which health and social care professionals can deliver structured physical activity brief advice to those within their care" and provided a list of how this might be achieved, including signposting to local physical activity opportunities and "refer to a structured Physical Activity Referral Scheme where such opportunities exist"viii.
However, the NPAP delivers structured physical activity brief advice to those already receiving care and we believe there are two issues with achieving this—
There is a lack of awareness among prescribers as to the opportunities which exist and no detail as to how this is being addressed; and
The lack of comprehensive coverage of Physical Activity Referral Schemes.
We request detail of how reliance on the National Physical Activity Pathway addresses both primary prevention, alongside reactive interventions from health and social care professionals.
We are concerned at the gap between the Scottish Government's expectations and impression of what is being delivered regarding social prescribing and realistic medicine and what is happening in GP practices.
We recommend the Scottish Government develop and implement a monitoring framework for the evaluation of NPAP. This should include regular reporting and provide comparable information across Health Boards.
We ask the Scottish Government to provide full detail of how boards are being supported to implement NPAP and how they are raising awareness of NPAP, "e-learning opportunities such as Raising the Issue of Physical Activity, and resources such as Moving Medicine"viii.
We ask the Scottish Government to provide specific details as to how awareness of social prescribing options will be raised with GPs and other primary care health care professionals to ensure they are aware of and utilising existing evidence on the benefits of specific activities for specific ailments, combined with access to detail of local initiatives.
In the Cabinet Secretary's responseviii to our reportiv on social prescribing, she indicated resources are being allocated to build capacity and expertise within healthcare to improve referral routes and breakdown barrier to referral. She said NHS Health Scotland was working to co-produce Quality Standards for Physical Activity Referral with academics and providers, and highlighted the work of the European funded mPower project to develop wellbeing plans for people over the age of 65. She added this project would also allocate funding to examining how access to activities in rural areas could be improved.
We would welcome more detail on how the European-funded mPower project can lead to increased capacity in the voluntary sector and improve access in rural areas.
She also referred to a community scheme run in collaboration with the British Heart Foundation on blood pressure management.
We were pleased to hear of the preventative action being undertaken as part of this project and request detail of whether this work will be rolled out across other areas. We note that “From 2020 the project will also explore how small pockets of funding can lead to increased capacity in the voluntary sector and support community transport options to improve rural access to activities.”
We seek confirmation of when the Quality Standards for Physical Activity Referral will be complete and in use by practitioners.
We were pleased to learn of the preventative action being undertaken as part of the mPower project and request further detail of whether this work will be rolled out across other areas. We also seek clarification of the funding for this project post 2020 and whether the Scottish Government will commit to matching funding that would previously have come from the European Union. Furthermore, we request details of how this project can lead to increased capacity in the voluntary sector and improve access in rural areas.
We would welcome more detail on how the Scottish Government is measuring or assessing effectiveness of the initiative on blood pressure management in the community, including if there are plans to introduce it in other areas.
We are also aware coverage of activities across the country is not uniform, as highlighted by Adam Stachura, Head of Policy and Communications, Age Scotlandi, and that the programmes being prescribed have to be appealing in order to work. Matt Barclay, Director of Operations at Community Pharmacy Scotland saidi there was an issue with referral pathways to social prescribing only being available to some prescribers. Claire Fernie, Public Partner Volunteer, NHS Fifei noted where activities were available, capacity was often an issue.
The response from the Cabinet Secretary to our report highlighted a number of examples of work intended to build capacity and capability of community organisations. However, we are unclear if there is a plan or planned approach to ensure this happens. The response also highlighted the work underway on the ALISS database. However, evidence received as part of our inquiry indicated that this database is out of date, under-resourced and not fit for purpose.
We would welcome more detail of action plans to increase or prioritise activity in community organisations. In particular, we are interested to hear more about what targets there are to achieve this and how they will be monitored and evaluated.
We would appreciate further detail on what plans and timescales are in place to address issues identified with the ALISS database, including what the process is to involve local people and Integration Authorities in this work and how the information contained in this database fits into the proposed health and social care digital platform.
The Cabinet Secretary for Health and Sporti was keen to emphasise the balance required to be struck between national policy and the local initiatives on which success would depend, but said a "national drive" was neededi.
We recommend the Scottish Government provide detail of how social prescribing can be uniformly and comprehensively embedded across the country, without detriment the local initiatives on which success may depend.
Inequality
A prevalent theme in our inquiry on social prescribing was inequality of access, both to preventative schemes and to social prescribing as a cure.
We are encouraged by the Scottish Government’s commitment to make sport and physical activity accessible to all. The responsei to our report indicated a range of activities to tackle inequalities, including work with sportscotland.
We would appreciate further detail on the aims and targets the Scottish Government is hoping to achieve through this work, alongside how the Scottish Government is measuring if this work is making a difference to people’s lives.
In our report we recommended a significant proportion of each Integration Authority budget be spent on commissioning local services to increase physical activity levels. We further recommended most of this investment is spent in the most deprived areas. In the Scottish Government's responsei, steps were highlighted which all Integration Authorities are required to undertake to ensure resources are spent in a way that improves the health and wellbeing of their community.
We would appreciate detail on how the Scottish Government are measuring and evaluating whether the resource allocation by Integration Authorities is leading to improvements in health and wellbeing in communities. We wonder whether this will be monitored through the Fairer Scotland Duty and seek clarification on whether there are any plans to issue guidance to Integration Authorities in this regard.
The responsei set out the commitment in the Programme for Government 2019-20 to develop community wellbeing services and supports across Scotland for 5-24 year olds.
We would welcome further detail on the timescales for this work, plans for how it will be achieved and what further action Scottish Government is taking to promote social environments, community assets and local connectedness.
Inquiry into Social Prescribing
In the Scottish Government responsei to our report following our inquiry onto social prescribing, a number of questions arose for us. While not directly linked with medicines, they do represent important factors in the wider social prescribing agenda and we take the opportunity of this section of this report to seek further details.
The responsei detailed how the A More Active Scotland action planiii “demonstrates how this evidence is recognised and informs action across Ministerial portfolios, setting out how the Scottish Government and a range of partners…are working together to achieve our shared vision of a Scotland where people are more active, more often”i.
The Plan outlines that the data for new indicators within the set that will become available from autumn 2018. It also outlines that these will be published on the Scottish Government Active Scotland Outcomes Framework web pages.
We would welcome an outline of how Scottish Government are monitoring and measuring results of the More Active Scotland action plan, and the outcomes this has achieved to date.
We were informedi sportscotland undertook an audit of community access to school sport facilities in 2013. This audit found that while 89% of school sport facilities were available for public use, there was considerable variation in actual levels of utilisation. Available for use is irrelevant if not actually used, accessible or affordable and we question what action is being taken to ensure public facilities are all of these things.
We would welcome a more recent update on this since 2013 as well as confirmation if there has been any research, examination or reason why there was considerable variation in actual levels of utilisation.
We are pleased to hear that the Scottish Government has committed to seek to offer three-year rolling funding for third sector organisations. We are aware that three-year contracts are a welcome start to providing stability for organisations.
We would welcome further detail on when the Scottish Government will start allocating three-year contracts, and timescales for ensuring this happens for all organisations.
We were informed the “Scottish Government is aware of and welcomes a number of positive examples where Integration Authorities are investing in physical activity programmes and working with local communities.” The response goes on to cite several examples of work where this is happening. However, the recommendation in our report referred to involving community and voluntary organisations in strategic commissioning.
Strategic commissioning is about planning and delivering services and support for people. It includes identifying the needs of individuals and communities, enabling people to decide what will best address those needs and working together with agencies to put the right services and support in place.
We are unclear whether the examples given came about through a strategic commissioning or a regular commissioning process and would welcome further detail on how these initiatives were planned and implemented. We would also welcome further detail on whether investment/provision and strategic planning processes can be reviewed as part of Integration Authority strategic plans going forward.
Social Prescribing - conclusions
We believe the case has been repeatedly made for social prescribing to have parity with medicine prescribing and are of the view it is just a case of making it happen.
The response to our report on our inquiry on social prescribing from the Cabinet Secretary said—
"I agree with the Committee that there is significant potential to increase the pace and scale of this work across Scotland, building on examples of best practice in delivery. We will therefore establish a Working Group to identify and communicate such examples of best practice, and coproduce resources for practitioners in the many roles which make up the overall system."i
We request further detail on timescales, proposed membership and remit for the Working Group.
Realistic Medicine

The concept of realistic medicine was supported by those who responded to our call for views and they highlighted the reasons why this approach to healthcare, including use of the 5 questionsi, can help improve the clinical and cost effectiveness of medicines prescribed.
The questions are—
Is this test, treatment or procedure really needed?
What are the potential benefits and risks?
What are the possible side effects?
Are there simpler, safer or alternative treatment options?
What would happen if I did nothing?
Dr Scott Jamieson of the Royal College of GPsii, the University of Strathclydeiii and several other responses to our call for evidence suggested conversations with patients to explain and discuss the value of medicines were vitally important, and there was widespread support for the use of the realistic medicine 5 questionsi. Reasons for this included—
To maximise the effectiveness of a medicine or a switch to another medicine, a patient must be invested in it. This included ensuring they were comfortable with multiple switches as more cost effective products became available (including generics or biosimilars, the benefits of which are described in the PRESCRIBING GENERICS AND BIOSIMILARS section of this report);
Avoiding situations where patients request medicines which are not the most cost or clinically effective;
Failure to have meaningful discussions with patients on their medicines results in waste, as patients are not engaged with the process and may not adhere correctly to the prescription. The importance of health literacy to adherence was emphasised to us from several sources. Dr Scott Jamieson of the Royal College of GPs said "If a patient does not know why they are taking a medicine or what its intended benefit is, that is a failure of the system. When we have time to ensure that realistic conversations are being had with patients, and the positives of taking their medicine are being explained to them, they will be more likely to value the medicines and take them."ii;
No medication or an alternative may be the better options; and
Good knowledge and understanding of ailments and medicines taken could help those with long-term conditions manage them better, and Dr Jamieson discussedii software options in this regard.
Matt Barclay of Community Pharmacy Scotland spokeii of the need to be natural with patients and root the conversation within their realm of experience in order to secure better outcomes. The British Medical Association (BMA) called for a "national conversation"iii with patients on realistic medicine. Adam Stachura, Head of Policy and Communications, Age Scotlandii said many people may not know they have the "right or are empowered" to be involved in discussions on their treatment. Claire Fernie, Public Partner Volunteer, NHS Fifeii, said those with long-term conditions may be more involved in discussions on their care. She added some respondents to questions on the realistic medicines programme had an expectation the doctor would know best.
We also heard viewsii that prescribers could be doing more to ensure patients had the time to process information provided by prescribers.
On training in having realistic medicine discussions with patients, Dr David Shackles of the Royal College of GPs told us—
“Given the time pressures, patient enablement is difficult to do, but it is something that we need to encourage our trainees to manage. However, the pressure on training means that elements such as patient enablement or motivational interviewing to help our patients are being squeezed out of already busy training schedules.”ii
He later told us—
"RCGP will continue to support clinicians to carry out medication reviews through the provision of high-quality training which focuses on medicines management"xiii
We inquired as to the training provided in this area and the General Medical Council informedxiv us of how this is carried out, including guidance currently being developed and plans to "help" GPs to follow this. The Royal College of GPs suggested training for GPs was not long enough in comparison with other medical specialities and said—
"RCGP would also like to see an increased proportion of training during this time spent in frontline general practice, where trainees will be able to gain greater experience of core general practice, including medicines management."xiii
Although Dr Jamieson of the Royal College of GPsii said he felt "tick box" exercises such as the quality outcomes framework (QOF) (which required GPs to fulfil certain tasks and demonstrate that) undermined the discussions with patients, we consider there needs to be a way to ensure this is happening and has proposed in the REVIEW OF PRESCRIPTIONS section of this report this should be through the GMS contract.
We are also not clear as to how in-depth the training in this area is. The Royal College of GPs have suggested there is not enough GP training time dedicated to a patient focused approach to issues such as reviews of medicines according to the principles of realistic medicine. The General Medical Council have stated doctors must demonstrate skills in history taking, diagnosis and medical management, as well as communication with patients and others. The emphasis placed on this element of GP training is not clear. The body had also noted it has produced guidance on the realistic medicine agenda which they encourage clinicians to follow.
We are once again struck by the void between the expectations of the Scottish Government and what GPs are actually delivering.
We recommend the Scottish Government consider use of the GMS contract as a tool to go beyond mere encouragement of its realistic medicine agenda.
Prescribing generics and biosimilars
Generic medicine

In its 2013 report, Audit Scotland noted there was "scope to make potential annual savings of up to £26 million without affecting patient care"i and one of the ways to achieve this was to increase prescription of generic drugs.
Audit Scotland said the Scottish Government Effective Prescribing team had helped boards reduce costs on medicines by "implementing electronic prompts for prescribers, to encourage them to use generic medicines and lower-cost alternatives."ii
Biosimilars
Biosimilars were described to us as—
"...copies of biologic medicines. Biologic medicines are made from living organisms, so they are inherently a bit variable as they are from different cell lines. The regulator reviewed and scrutinised those medicines and said that they have comparable quality, safety and efficacy. The benefit of biosimilars is that, because they are a copy of a product that was on patent and had no competition, we now have competition and significant savings for the first time. In that area, we work closely with boards to ensure implementation and uptake of the most cost-effective biologic products. Over the next few years, more of those products will be coming off patent and we will have to continue to work to control spend."i
Benefits of prescribing generic medicines and biosimilars
Generic prescribing rates have been increasing each year in Scotland since 2009/10 according to NHS National Services Scotland Information Services Division (ISD). In 2018/19, 84.3% of prescriptions were generic drugsi.
Audit Scotland highlight the cost effectiveness of prescribing generic medicine and biosimilars in the organisation's report "NHS Scotland in 2019"ii. By way of example, the report notesii NHS Grampian achieved a saving of £3.5m in 2017/18 compared with the year before through increased prescription switches to biosimilars.
Rose Marie Parr, the Chief Pharmaceutical Officer, Scottish Government, said "Across Scotland, millions of pounds have been saved by changing people not just to generics but to biosimilars, which are similar to the active ingredients that the patients used previously. There are areas in Scotland in which we can be nimble about getting the best price for the NHS."iv Dr Brian Montgomery usediv the example of switching from branded statins to generic versions used by other health boards to illustrate how a £1m saving could be achieved in one move.
Governance of generic and biosimilar prescribing
Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS National Services Scotland (NHS NSS) said—
"The regulator reviewed and scrutinised those medicines and said that they have comparable quality, safety and efficacy. The benefit of biosimilars is that, because they are a copy of a product that was on patent and had no competition, we now have competition and significant savings for the first time. In that area, we work closely with boards to ensure implementation and uptake of the most cost-effective biologic products. Over the next few years, more of those products will be coming off patent and we will have to continue to work to control spend."i
Dr Ewan Bell, National Clinical Lead, Area Drug and Therapeutics Committee (ADTC) Collaborative, Healthcare Improvement Scotland (HIS), said "There is a good news story. The ADTC collaborative, which sits within HIS, was responsible for developing a prescribing framework for moving patients from biologics to biosimilars. The framework tries to ensure consistency of approach throughout NHS Scotland, and has saved tens of millions of pounds. There is an opportunity, as biologics come to the end of their patents, to move patients from biologics to biosimilars that have the same effect. There is a high rate of generic prescribing within NHS Scotland, and that needs continued vigilance. There are good things happening, supported by stakeholders including the ADTC collaborative."i
Cancer Research UKiii proposed a drive to increase use of biosimilars, akin to that being undertaken in NHS England and the British Generics Manufacturers Associationiii said programmes to encourage switching to biosimilars should be prioritised.
While welcoming the progress made in prescription of generic medicine and biosimilars, we urge the Scottish Government to maintain momentum and explore all opportunities for sharing of best practice and further savings in this area.
Prescription charges
Charges for prescriptions were removed in Scotland in 2011. There has been a 20.5% increase in the number of items dispensed in the first 10 years of operationi.
We were interested in how removal of prescription charges had altered perceptions of prescriptions for low cost items such as paracetamol. In general, responses to this query suggested the removal of prescription charges was in the main a good thing, including when measuring cost effectiveness.
Matt Barclay, Director of Operations at Community Pharmacy Scotland noted prescribed paracetamol was used by patients to manage chronic pain conditions, and if prescriptions for this were not available, the alternative would be more expensive. He added "....with regard to patients who need analgesia for significant acute conditions or chronic pain, I would focus more on the overall value than the individual cost of the medicine, although I appreciate that the differences in cost could be significant."ii
We were interested in whether prescriptions for low cost items could be removed altogether and prescribers could hand out medicine on the spot. The British Dental Associationiii noted the advent of removal of prescription charges had seen more demand for specialist toothpaste and suggest savings could be made if it could be purchased directly from the dentist. They also proposed limiting the amount of prescriptions of toothpaste per patient to achieve savings.
The idea of including the cost of medicines on prescriptions was also of interest to us. NHS Grampian Area Drug and Therapeutic Committee (ADTC)iii said there was a need to communicate the value of medicines to patients, as free at the point of receipt did not mean cost neutral.
Prescribing non-licensed medicines
We were interested in prescriptions for unlicensed medicines.
There is however a difference between prescribing a medicine which has no licence at all, and prescribing one which has a licence for a different indication, known as 'off-label' prescribing. Alison Strath, Principal Pharmaceutical Officer, Scottish Governmenti and David Coulson, Assistant Director of Pharmacy, NHS Taysidei described 'off-label' prescribing, with the latter saying—
"It means that we have a medicine that has been licensed through our regulatory body, but the prescriber is not using it for that particular indication."
There appeared to be confusion as to whether medicines with no licence could be prescribed. Dr Brian Montgomery saidi the General Medical Council (GMC) required doctors to prescribe licensed medicines. The British Medical Association (BMA) saidiv it was "rare" to find a doctor who would prescribe off formulary or suggest unlicensed medicines. Dr Scott Jamieson of the Royal College of GPsi noted the guidance on such prescribing highlighted the crux of the issue - the most efficacious and cost effective drugs to treat a particular ailment may not be licensed for that indication, leaving the prescriber exposed to risk in taking an individual judgement.
Faced with various views, we sought clarification from the Scottish Government.
The Chief Pharmaceutical Officer, Rose Marie Parr,i told us each health board had its own governance system and guidance for prescribers on use of unlicensed drugs, which came from area drug and therapeutic committees (ADTCs). The Cabinet Secretary for Health and Sport, Jeane Freeman, noted there may be confused or varied interpretation of governance and said "we might need to look at how well understood the current governance process is"i. She also undertook to ask the ADTC collaborative—
"...to see whether there is any way for us to streamline the process in any way, and to ensure that prescribing clinicians know what the current route is and what they have to do in order to prescribe off-label—they are not forbidden from doing that."
Alison Strath, Principal Pharmaceutical Officer, Scottish Government, informed us of work being funded by the Scottish Government and supported by Healthcare Improvement Scotland (HIS) to "test how we might use some medicines off-label and how we might create a governance structure for that. We have started by looking at cancer medicines because a number of those fell into that category. What we learn from that will help us to think about how we link our policies."i
We sought details of the work, including the remit of the project, intended outcomes and timescales for completion in correspondenceix to the Cabinet Secretary.
Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS National Services Scotland (NHS NSS), toldi us NHS NSS was able to procure unlicensed medicines for use within the NHS in Scotland.
We welcome the commitment from the Cabinet Secretary to liaise with the Area Drug and Therapeutic Committee collaborative on how guidance for off label prescribing could be developed and shared with prescribers to ensure they are aware of how to achieve the most clinical and cost effective outcomes, and look forward to an update on how this is progressed.
Polypharmacy
Dr David Coulson, Assistant Director of Pharmacy, NHS Tayside, said "It is also about understanding the polypharmacy challenge. Why a patient is taking 10 medicines should be questioned; it might be absolutely fine for one patient but inappropriate for another. From community pharmacy, to pharmacies working in general practice, to nursing teams, we all have a responsibility for that, and for managing the pill burden and the value that we get from resources."i
The Royal College of Physicians of Edinburgh saidii opportunities to address polypharmacy were not always taken and in some circumstances deprescribing could save money and improve outcomes.
The Cabinet Secretary for Health and Sport, Jeane Freeman, said—
"Until 2012-13, there was an annual volume increase of 3 per cent. Since the introduction of the first polypharmacy guidance in 2012, the rate of volume increase has fallen each year."i
Non-medical prescribers
Eileen McKenna, Associate Director Professional Practice, Royal College of Nursing Scotlandi and Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotlandi spoke of the benefits of non-medical prescribers, including nurses and pharmacists respectively. They identified issues surrounding education and qualifications in this regard. Campbell Shimmins of Community Pharmacy Scotland spoke of a scheme in NHS Ayrshire and Arran, details of which were sentiii to us by the health board, aiming to have independent prescribers in every pharmacy. This service has been a pre-curser to the Pharmacy First scheme and has seen patients present at their pharmacy, rather than GP surgery for common ailments.
Prescribing - conclusions
The influence of prescribers on the clinical and cost effectiveness of medicines was a prominent theme throughout evidence received on prescribing.
The use of and adherence to a formulary is key to this and we look forward to hearing more on the Government's vision for a single national formulary or an alternative formulary approach such as a condition specific pathway (as indicated previously in the report). We also want to see the opportunity taken to improve governance surrounding non-pharmaceutical interventions. We caveat this by suggesting a national formulary must address concerns about the loss of benefits provided by a local system.
We believe the GMS contract should be used to ensure strategies on medicines and non-pharmaceutical interventions, such as social prescribing, are achieved and carried out in practice. Social prescribing remains a huge missed opportunity and we look forward to further details as to how the Scottish Government will make the urgent progress needed in this area.
Reviews of prescriptions were a further area where we have recommended prompt attention is required, and again we have sought detail from the Scottish Government as to how the new aspects of the GMS contract will assist with ensuring regular and comprehensive reviews are taking place for those on long-term prescriptions. Repeat prescription management could be similarly improved.
We were disappointed prescribing in secondary care has fallen behind and by the evidence regarding the implementation of the HEPMA, suggesting there is no leadership or strategic oversight of this project. Again, we have recommended this cannot continue. We are also concerned at evidence suggesting prescriptions in secondary care were leading to delays in patient discharge from hospital and suggest this requires urgent consideration.
Non-pharmaceutical interventions, including social prescribing, require more scrutiny and given greater parity with medicines governance.
Prescriptions of generic drugs and biosimilars represents a large proportion of prescribing in Scotland and therefore achieves savings. We have suggested momentum in this area be maintained by promoting best practice. Similarly, we have suggested governance surrounding prescriptions of unlicensed medications could be improved to ensure opportunities are being maximised to make the best cost and clinically effective decisions.
Dispensing

Once a prescription has been provided for a patient, it is then presented to a pharmacist to dispense the medicine.
According to the Scottish Parliament Information Centre—
"In Scotland, patients may have their medicines dispensed in hospital by a hospital pharmacist employed by the NHS, or in the community by a community pharmacist or dispensing doctor practice. In order to dispense NHS prescriptions, a pharmacy must be entered onto the NHS board's pharmaceutical list. This involves an application to the board and consideration by its Pharmacy Practices Committee. Pharmacies are included on the list when it is considered necessary or desirable in order to secure adequate provision of pharmaceutical services."i
This section of the report will consider evidence presented to us on the best way to ensure clinical and cost effectiveness as this stage. We have examined—
The role of community pharmacy, including the new Pharmacy First Initiative;
Automated dispensing;
Dispensing in care homes; and
Online pharmacy.
Community pharmacy contract
We are interested in how the community pharmacy contract can influence the clinical and cost effectiveness of medicine interventions.
Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland said—
"We are always talking to the committee about the additional value that pharmacists bring through their conversations with patients, oversight activities and so on. Up to this point, it has been difficult, because of lack of IT and how the contract is developed over time, to prove that added value by quantifying and recording it. However, we are getting closer to the point at which we can build a really good evidence base, ensure that there is good visibility for the great work that goes on around dispensing, and incentivise pharmacists to engage with that and be consistent in it."i
We look forward to the Royal Pharmaceutical Society in Scotland presenting evidence to support its claims of added value. We are sympathetic these should exist but recommend the profession start collecting the necessary evidence which we imagine would, if substantiated, be positively received by the Scottish Government in future contract discussions.
We are interested in plans to evaluate and monitor the first year of operation of the community pharmacy contract, especially in light that this will not be renegotiated for three years, rather than the previous annual renewal. We sought the view of the Cabinet Secretary for Health and Sport on this issue in correspondenceii. We also asked whether there were plans to include further incentives to drive better pharmaceutical care, including data retention.
We were also interested in whether the community pharmacy contract created a perverse incentive for community pharmacists to dispense medicine rather than discuss alternatives as they will be recompensed for the drugs. Campbell Shimmins of Community Pharmacy Scotlandi suggested that may previously have been the case but the nature of the contract was changing to include more service delivery elements such as Pharmacy First. Angela Timoney, Director of Pharmacy, NHS Lothiani said the contract was moving towards "professional fees for their services". The Chief Pharmaceutical Officer, Rose Marie Parr, said—
"We want community pharmacists to make a wider range of patient-focused interventions and come away from supply dispensing, although not completely, to perhaps look at other ways to do that and be much more up front with the patient in talking about their medicines and trying to reduce some of the harm that can happen."i
She suggested there were two "streams" to that work—
Pharmacy First; and
Support for patients with long-term conditions
Minor Ailment Scheme and Pharmacy First
Pharmacy First is the new title for the Minor Ailment Scheme, which "sees community pharmacists able to give advice to and if necessary treat or refer patients..."i with uncomplicated conditions.
Where previously the eligibility criteria for the minor ailments scheme was limited, the scheme will be widened to cover everyone and the original April 2020 launch for this has been delayed due to the COVID-19 crisis.
Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government, emphasised the shift in the contract from simply remunerating dispensing to a focus on other services provided by pharmacists—
"We have to think about what the Scottish drug tariff is there for. It reimburses our community pharmacists for the drugs that they have already bought—they have taken the risk of buying them—but it also pays for aspects of the community pharmacy service. We have a contract agreement with Scottish pharmacy contractors around acute prescribing—everyone will see that when they take a prescription to a pharmacist—and around the pharmacy first service, which will be rolled out in April 2020. Pharmacists will be able not just to focus on self-care, but to look at pharmacy first treatment, which will include antibiotics for urinary tract infections and some skin problems. The point is to avoid having to go to a prescriber—a GP or someone in accident and emergency—and to allow community pharmacy to take on some of that burden. The issue for me is less about what drugs are within the tariff and more about how they are used and reviewed.ii"
Jonathan Burton of the Royal Pharmaceutical Society in Scotlandii, was enthusiastic about the new Pharmacy First service and highlighted the role community pharmacists could play in providing a consultancy service which may mitigate the need for people to make a GP appointment.
Campbell Shimmins of Community Pharmacy Scotland cited research into the impact of the minor ailment service which he described as "overwhelmingly positive"ii. Along with Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clydeii and Jonathan Burtonii, heii suggested more publicity, education and communication with the public will be required when the scheme is extended to all.
We are interested in how the Pharmacy First initiative will be evaluated and monitored and sought the view of the Cabinet Secretary for Health and Sport on this issue in correspondenceviii.
We support this development although we are unclear whether positivity and enthusiasm for the Pharmacy First scheme among professionals may not match with public awareness of the scheme as was reflected in discussionsii with stakeholders. We are interested in how the new Pharmacy First scheme will be advertised and sought the view of the Cabinet Secretary for Health and Sport on this issue in correspondenceviii. We also sought details of the expected demand.
Pharmacy and the General Medical Services Contract
Community Pharmacy in GP surgeries - Pharmacotherapy
The new GMS contracti has introduced co-location of community pharmacists within GP surgeries to provide advice and prescribe and dispense medicine, although it was highlightedii to us this form of working is not new.
The University of Strathclyde saidiii access to pharmacists in GP practices was currently limited—
"An evaluation of the GP practice-based pharmacists programme has been commissioned to capture the learning and inform any models for future practice developments."iv
The Chief Pharmaceutical Officer, Rose Marie Parrii, said job roles would have to be more flexible in the future.
The Cabinet Secretary for Health and Sport, said—
"The introduction of the pharmacotherapy service in around 70 per cent of our general practices as a result of phase 1 of the GP contract means that pharmacists and pharmacy technicians are now embedded in the general practice team, who can provide medication management systems, including formulary compliance, hospital out-patient requests, medicine reconciliation and repeat prescribing management. They can also provide polypharmacy and medication reviews, including of high-risk medicines, and take on the management of people with more complex multiple conditions, which involves taking decisions with individuals on the use of their medication and, where appropriate, monitoring and adjusting treatment prescriptions. By taking on that role, those pharmacists are improving clinical outcomes for people, reducing the workload of GPs and freeing up the capacity of others to focus on people with undifferentiated illness or other complex needs."ii
The impact of the pharmacotherapy aspect of the contract was discussed in several representations to us throughout the inquiry and themes arising included—
Relationship between pharmacist and community;
Impact on the pharmacy workforce;
Skills and training; and
Ability to work remotely.
Relationship between pharmacist and the community
The Company Chemists Associationi suggested "pharmacists within community pharmacy settings are ideally placed to work in collaboration with GP teams" noting the enhanced relationship between those working in the community and the public. The theme of community pharmacy work with the public was prevalent throughout our work, with several organisations extolling the benefits of the discussions people have with their pharmacist, including—
Development of a relationship of trustii
Ensuring patients know how to take their medicine and what the benefits are, as this helps with safety, adherence and waste reduction; and
Allowing patients to make an informed decision about whether to take a prescription.
While much was made of the importance of conversations between pharmacist and patients, and the benefits of the close relationship which can form, it was unclear to us the extent to which—
pharmacists will contradict the advice of prescribers and what influence such discussions may have on whether people take their prescriptions; and
Information gleaned from such conversations is recorded and put to use.
Campbell Shimmins of Community Pharmacy Scotlandii said there was not a conflict between the advice of prescribers. Both Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clydeii and Angela Timoney, Director of Pharmacy, NHS Lothianii, spoke of the good working relationships between GPs and pharmacists. Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotlandii quotedii a study suggesting around 5% of prescriptions were technically inaccurate to highlight an area in which the pharmacist was an important safety net.
However, we were concerned that in all the evidence we have taken on the importance of discussions between community pharmacists and patients at the point of dispensing, there is no route for processing the information offered by patients. Nor is there any indication of the extent to which these conversations are happening. Jonathan Burtonii stated recording of discussions was down to the professional judgement of the pharmacist but, in describing situations where he sent Post It Notes to GPs, felt communication could be improved. We question the professional judgement involved in recording important information relating to patient health in such an ad hoc informal manner.
Community Pharmacy Scotlandix highlighted the use of paper forms for this purpose adding uptake was not comprehensive. The Royal Pharmaceutical Society in Scotland later provided us with its view that in order for information to be recorded, it would have to be mandatedx. We are concerned at professional people and bodies adopting such a position.
We are also surprised the gathering of data on interactions with patients within community pharmacy is not a condition of the community pharmacy contract.
Campbell Shimminsii suggested visiting people's homes was a key way to understand how they were taking their medicines. Although he acknowledged this is not a regular occurrence, he also saidii there was no formal process for doing so. Dr David Shackles of the Royal College of GPsii spoke of initiatives where pharmacy technicians visited the homes of patients.
We are keen to understand more about the governance of community pharmacists visiting the homes of patients. Community Pharmacy Scotlandix suggested the more pertinent issue was the absence of a pharmacist from a pharmacy which impeded the normal business of a chemist from being carried out. While according to CPS, the regulations covering this are reserved, we are interested in whether such practice is safe and covered by suitable governance structures such as the PVG scheme.
We ask the Scottish Government for full details of the governance structures surrounding pharmacists work in the homes of private individuals, recognising this is an infrequent occurrence, and how this is covered in the Community Pharmacy Contract.
We also ask the Scottish Government to advise what aspects of data collection are covered in the new pharmacy contract.
We recommend the Scottish Government, as a matter of urgency, formalise the systems for sharing information between prescribers and dispensers. This should be done in collaboration with GPs and pharmacists.
We heard much from pharmacists about the personalised services they offer from the heart of communities, including making house calls and ensuring people received their medicines in extreme weather.
However, we are also aware some pharmacists have started charging for deliveries although these charges can be avoided if prescriptions are booked online. Adam Stachura, Head of Policy and Communications, Age Scotlandii suggested this was detrimental to the care of the 0.5m people in Scotland who do not have access to the internet. Claire Fernie, Public Partner Volunteer, NHS Fifeii described this as a barrier to access.
We recommend the Scottish Government consider the impacts of charges such as this from private companies in preparation of the next Community Pharmacy contract and in the meantime liaise with the organisations as to how these could be mitigated and removed.
We recommend the next iteration of the Community Pharmacy contract takes account of compensation for pharmacist delivery of prescriptions.
Impact on the pharmacy workforce
Several organisations, such as the Company Chemist's Associationi, McKesson UKi, the Royal Pharmaceutical Society in Scotlandi and Community Pharmacy Scotlandi, noted the impact of pharmacists integrating within GP surgeries, which caused a reduced workforce pool to be available to the community pharmacy service. It was proposedv the speed of this had compounded the issue and we heard calls for further work force planning to ensure adequate numbers of both pharmacists and pharmacy technicians.
Community Pharmacy Scotland suggested the inclusion of pharmacists within GP practices was welcome, but the key benefit was communication which they felt could be improved between GPs and pharmacists working in the community. They also proposed that different models were available indicating future discussions on this were imminent. The Chief Pharmaceutical Officer, Rose Marie Parrv, said there was a need to work flexibly across job roles in the future which would be a new way of working and we sought details of this from the Cabinet Secretary for Health and Sport in correspondencevii.
When questioned on the impact of the GMS contract on the pool of personnel on which community pharmacy had to draw, Bryan Lamb, Head of Pharmacy Branch, Scottish Government said—
"As part of the three-year agreement that we have entered into with Community Pharmacy Scotland, we are looking to introduce a new independent prescribing career pathway as well as a foundation programme, which is about encouraging people to come back into community pharmacy to practise. They will be able to maximise not only their patient interaction skills but their medicines knowledge, and they will be able to provide patients with long-term treatment. That is a key change in how we are bringing people into the network to sustain community pharmacy."v
The Scottish Government stated that in order to deliver the new GMS contract, it would—
"...increase the pool of qualified pharmacists to provide the pharmacotherapy service, additional funding has been secured to increase the number of pharmacist training posts from 170 to 200 per year from 2018/19. This will ensure that there is sufficient capacity to deliver the pharmacotherapy service within the proposed timescales."ix
However, in light of the concerns heard throughout the inquiry from stakeholders, we are concerned as to whether there are sufficient trained numbers of personnel available to deliver this. Indeed, Angela Timoney, Director of Pharmacy, NHS Lothian proposed adding pharmacists to the Shortages Occupation List (SOL).
In the REVIEWS OF PRESCRIPTIONS section of this report, we sought an update on progress towards delivering pharmacy support services in every GP practice in Scotland and we are also interested in whether the support for training programmes has been sufficient to "ensure"ix delivery of the "pharmacotherapy service within the proposed timescales"ix.
We ask the Scottish Government to provide an update on the training programmes which have been supported for pharmacists since the start of the current GMS contract. To include the numbers of students taking up places as compared to the numbers of pharmacists the Scottish Government envisaged were needed to "ensure that there is sufficient capacity to deliver the pharmacotherapy service within the proposed timescales"ix.
Skills and Training
Skills and training were raised with us throughout the inquiry.
The Royal Pharmaceutical Societyi noted there could be a greater role for Pharmacy Technicians, particularly in dispensing, but said a more formalised career path was required. The Royal Pharmaceutical Society in Scotland also suggestedi the correct level of qualified personnel should be assigned to the correct tasks to ensure efficiency, best value and more patient facing time for pharmacists.
It was also suggestedi investment in digital skills for pharmacists would be required as systems moved online and an increase in the number of pharmacists working in data scientists and clinical informatics roles. Angela Timoney, Director of Pharmacy, NHS Lothianiv, said the board had invested in developing its own workforce to combat shortages by funding places at Edinburgh college for technicians.
The Chief Pharmaceutical Officer, Rose Marie Parriv, indicated funding had been made available for pre-registration training and there were ongoing discussions with training providers on this.
Automation
In August 2017, the Scottish Government published "Achieving excellence in pharmaceutical care: a strategy for Scotland"i which included a recognition of the need to transform delivery of services through use of technology. They stated—
"We are committed to this transformative programme of work which includes automated technologies.i"
Advocates of automation suggested greater use of technology to dispense at scale would—
Improve safety and accuracyiii;
Save time allowing community pharmacists more time with patients;
Create "capacity within the system and specifically within GP practice"iii by reducing GP workload on repeat prescriptions;
"Optimise space available in the dispensary"iii;
"Avoid duplication"iii;
"Increase distribution safety standards"iii;
"Create safe and efficient repeat prescribing"iii;
Reduce the number of errors in the dispensing process; and
Save nursing timeiii.
Omnicell provided us with a detailed submissioniii extolling the virtues of introducing technology into the dispensing process with a strong focus on reducing errors and improving efficiency. As well as the many benefits for staff and the NHS, they cite the following issues which can arise for patients if errors occur—
"Medication errors can result in adverse drug reactions, drug-to-drug interactions, a lack of efficacy, sub-optimal patient adherence, impaired quality of life and a poor patient experience."iii
In response to questions on the Omnicell submission, Adam Osprey, Policy and Development Pharmacist, Community Pharmacy Scotland said "a computer is only as intelligent as what somebody tells it to do"xii. He also said one solution might not suit all types of pharmacy, noting robotic solutions may work in hospitals but not in community pharmacies.
On robotic dispensing, Omnicell said—
"Robots have the potential to manage stock rotation – reducing medicines wastage, handle high volumes of dispensing in community pharmacies, or dispensing “hubs”, and release pharmacists to develop and deliver patient-centred services."iii
Adam Ospreyxii, suggested robotic dispensing required huge scale in order to produce efficiencies. He proposed, however, other interventions such as barcoding on medicines could help ensure accuracy of dispensing.
While recognising the potential automation has to revolutionise dispensing, we question how much has been achieved so far in trials in Scotland. "Achieving Excellence in Pharmaceutical Care - A Strategy for Scotland" states—
"Within NHS Greater Glasgow and Clyde (NHS GG&C) the largest hospital pharmacy robotic installation in the world exists within a Pharmacy Distribution Centre and distributes nine million packs of medicines to 4000 destination points from the eastern boundary of Glasgow to Argyll in the west Highlands. The main hospitals within NHS GG&C also have dispensing robots which dispense medicines for individual people. The use of this technology has enabled a very much smaller pharmacy to be built in the new Queen Elizabeth University Hospital than would otherwise have been required and has enabled pharmacy staff working in that hospital, freed from dispensing, to be available at the bedside to assist people to achieve the best outcomes from their treatment with medicines. In addition, semi-automated medicines cabinets are being installed within hospital wards and departments. The Golden Jubilee has recently invested in this technology for the whole hospital and the benefits include releasing time for nurses to care for people and quicker access to stored medicines meaning that people can receive for example pain relief as required more quickly. Typically wards can store 20% less medication through use of these cabinets while still providing medicines to meet the people’s needs."i
The Scottish Government indicated evaluation of trials highlighted in the "Achieving Excellence in Pharmaceutical Care - A Strategy for Scotland"i were underway and we would welcome detail of these. Graeme Bryson, Director of Pharmacy, NHS Dumfries and Gallowayxii, noted a Scottish Government commissioned report on robotics in community pharmacies is due to report in the middle of the year.
We ask the Scottish Government to provide detail on the evaluations of pilots and trials undertaken by both the Scottish Government and health boards of automated dispensing so far in Scotland and details of the current work due to report in the middle of the year.
Care Homes
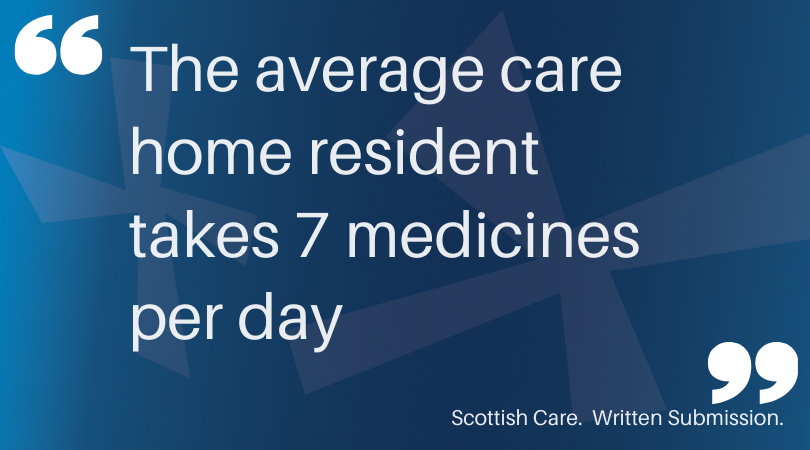
The issue of prescribing and dispensing medicines in care homes and the potential for reducing waste and improving efficiency was raised with us by several sources.
Researchers from the University of Edinburgh provided a detailed account of the potential for reduced waste and more efficient dispensing of medicines in care homes covering—
Consideration of the use of 'Just in Case' boxes;
Include a stock of medicines in care homes in the way hospitals and hospices do; and
Allow bulk prescribing in care homes.
Others were keen to see pharmacists have a direct role working within care home settings. We look at these suggestions in turn.
Use of 'Just in Case' boxes
The University of Edinburgh researchers provided the following explanation of 'Just in Case' boxes—
"Anticipatory prescribing in the community, known as ‘Just in Case’ boxes, were traditionally set up for people dying in their own home, using individualised patient prescriptions with the person’s name typed on each medication. They are recommended by the Scottish Palliative Care guidelines, and became part of policy in case of distress in the last days of life and saved calling out a GP at weekends/OOHs. Each ‘Just in Case Box’ contains ten ampoules of each anticipatory medication. The above best practice in end-of-life medication provision then became popular within the care home setting in order to save the care home organisation having to pay a ‘home office licence’ in order to have controlled drugs in the care home ‘as stock’."i
The researchers suggest the boxes result in a lot of waste, and pose problems with storage and pharmacist and staff time in preparing and checking the boxes. Medications which are not taken prior to a patient's death are returned and cannot be used again. Fife Area Drug and Therapeutic Committee (ADTC)i told us it was working with care homes to reduce avoidable returns.
A comparison of the two different approaches was provided—
| Just in Case Boxes | Stock |
|---|---|
| JiCBs are issued for use during last days of life | ‘as stock’ can be used during last days of life + for any ‘acute’ issue once prescribed by a GP/OOHs |
| JiCB are for individual people only | Used for any resident |
JiCB for each resident includes following medication:
| ‘as stock’ medication would include just one box of each medication i.e. s/c morphine; s/c hyoscine; s/c midazolam; s/c levomeprazine. |
| Time wastage for pharmacists creating JiCBs for each resident | Less time wastage for pharmacists as only supplying one box of each medication per care home |
| It is rare that more than two or three JiCB medications used - all remaining ampoules then returned/destroyed by pharmacist once resident dies | No names on ‘stock’ medication so don’t need to be destroyed unless ‘out of date’ |
| Between 30-50% of residents in a nursing care home will have JiCB prescribed – so need of checking each JiCB twice a day + need for large storage cupboards. | Simple checking of four boxes of ‘as stock’ medication twice a day. No need of large storage cupboards. |
| Don’t have to pay for Home Office Licence | Currently independent care home organisations have to pay for a Home Office Licence to hold medications ‘as stock’ but independent hospices don’t |
Stock of Medicines
Dr David Shackles of the Royal College of GPsi suggested there were circumstances, such as care homes, where stocking medicines may be a good way to reduce waste, a notion supported by Scottish Careii.
It was suggested that care homes use 'Just in Case' boxes to circumvent the requirement to pay to hold a licence from the Home Office to have a stock of medicines. We wrote to the Home Secretary, Priti Patel, to inquire as to why this was the case and received a responseiii from the Minister of State for Crime and Policing, Kit Malthouse. This notes the area is regulated by the Misuse of Drugs Regulations 2001 and highlights exemptions to the requirement to hold a licence—
"Care homes which are wholly or mainly maintained either by a public authority out of public funds or by a charity or by voluntary subscriptions
Care homes not satisfying these requirements are also able to hold controlled drugs in Schedule 3 to 5 (inclusive) without a Home Office licence, but they will need a controlled drugs licence for drugs in Schedule 2."iii
He stated there are no plans to review this legislation.
The Chief Pharmaceutical Officer, Rose Marie Parr, statedvi the Scottish Government suggested it was for each home to advocate to the Home Office for exemptions to the requirement to hold a licence. She explained the regulatory burden this would place on the individual home, as well as the governance and risk challenges such as lack of nursing staff and general workforce challenges faced by the sector, and improved margin for error when selecting from stock rather than individual patient's medicines.
Recognising the challenges associated with the proposed alternative to 'Just in Case' boxes, we believe this is an area worthy of further consideration as to how to reduce the associated waste.
We ask the Scottish Government how much wastage is produced from care homes returning medicine and at what cost to the NHS?
We recommend the Scottish Government consider how medicines could be held in care homes in a manner which does not produce the levels of waste caused by 'Just in Case' boxes.
We recommend the Scottish Government work with the Home Office to consider further the approach to licensing in care homes as well as the fees payable for licences for different types of organisation.
Bulk prescribing in care homes
The Royal Pharmaceutical Society in Scotlandi suggested bulk prescribing could be a way to eliminate waste, citing an example of nutritional drinks which if prescribed for one patient but not used, cannot then be kept by the care home for use by another patient.
NHS Tayside providedii us with detail of the model used by the board, in collaboration with care providers, to reduce medicines waste arising in care homes. A new protocol and pathway was developed, ensuring only appropriate medicines were returned to pharmacists and the results are shown below—
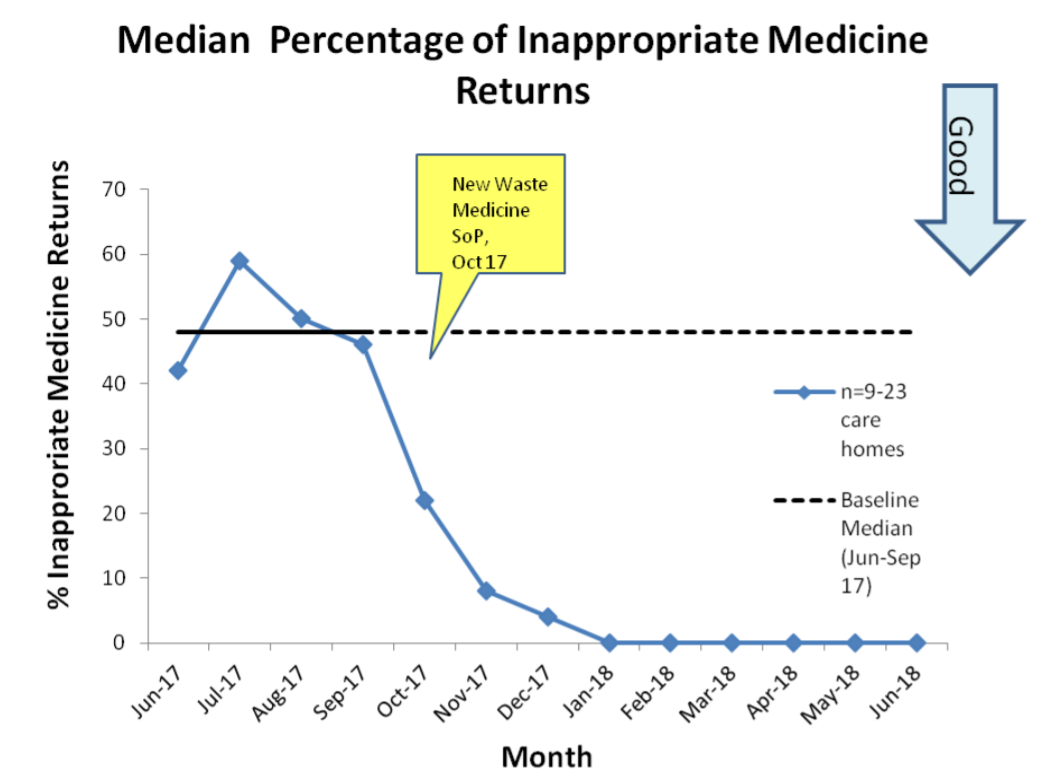
Pharmacists reportediii seeing a huge reduction in returned medicines, which they previously said had amounted to tens of thousands of pounds of waste every month. NHS Tayside suggested to us "If there are opportunities to influence and develop government policy related to medicines management within care homes, based on this effective work, there would be a significant and positive impact across the whole of Scotland."ii This approach was praised by Community Pharmacy Scotlandv.
We recommend all Health Boards consider the results of the work undertaken in NHS Tayside and we ask the Scottish Government who would be responsible for facilitating the roll out of similar schemes in every health board in Scotland.
Other health care staff's presence in care homes
According to the Scottish Government "There is also an important role for GP practice-based pharmacists, at the interfaces of the profession, working closely with hospital pharmacists, community pharmacists and care homes to ensure seamless care and reduce potential medication related problems and errors."i
Others, including Scottish Careii, also emphasised the role pharmacists could be playing directly in care homes.
AstraZenecaiii called for investment to allow community and GP pharmacists to work in care homes. The Royal Pharmaceutical Society in Scotland felt care homes should have more dedicated services from pharmacistsiii. The organisation proposed this would—
Reduce waste through more effective, evidence based prescribing;
Address inappropriate polypharmacy in care homes
They also called for consideration to be given to bulk prescribing in care homes to make efficient use of resources and Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotlandv thought this would help reduce waste by allowing care homes to draw on a supply rather than have items prescribed for individual patients. Community Pharmacy Scotlandiii also said pharmacists should be working within care homes and suggested guidance from the professional body would be helpful. Jonthan Burtonv suggested pharmacists should be permanent members of staff within care homes in order to optimise impact and support areas like polypharmacy review and training support for the pharmaceutical aspects of tasks undertaken by care assistants.
Dr Lewis Morrison, Chair of BMA Scotland, British Medical Association (BMA)v, advocated those in care homes were entitled to the same levels of care as others and said this would be achieved through a multidisciplinary approach.
Eileen McKenna, Associate Director Professional Practice, Royal College of Nursing Scotlandv, said there was an opportunity through the Transforming Roles programme to examine the role of nurses in nursing homes. Claire Fernie, Public Partner Volunteer, NHS Fifev, spoke of a pilot in Fife where GPs and Pharmacists went into care homes together, which she said reduced waste but also improved safety and wellbeing through formal reviews of medicines.
Scottish Careii proposed each care home should have a link pharmacist, noting recent work on linking dentists with care homes and suggesting a similar model should be considered by the Scottish Government.
We request detail from the Scottish Government on what learning from good practice elsewhere is undertaken in this area and how that is encouraged.
We seek the view of the Scottish Government on the views presented to us on making greater use of the skills of nurses in care homes.
We agree pharmacists should have similar roles in care homes to those in GP practices and seek detail of what the Scottish Government can do to make this a reality.
Scottish Government consideration of dispensing in care homes
The Chief Pharmaceutical Officer, Rose Marie Parr, detailedi further work on how medication is used in care homes in correspondence. She wrote—
"One of our five Pharmacy Clinical Leadership Fellows is undertaking work on improving the pharmaceutical care of residents in care homes, in line with Achieving Excellence in Pharmaceutical Care. Along with taking forward examples of best practice of pharmaceutical care in care homes for national implementation they are exploring—
The development of a care home specialist interest group.
The greater use of electronic Medicine Administration Record (eMAR) in a care homes.
The use the Medicines: Care and Review (M:CR) service in care home residents.
Examine the use of a palliative care assessment tool in care homes, and
Address the issue of improving waste reduction of medicines in care homes."ii
We note the exploration going on and would like to see that turned into action with the benefits that would accrue being delivered to patients and budgets. We request an update on when these proposed changes will be delivered in practice in care homes.
Online Pharmacy
We were interested in the regulation and operation of online pharmacy, although it was suggested by various witnesses this was not a prevalent issue in Scotland.
Campbell Shimmins of Community Pharmacy Scotlandi and Adam Stachura, Head of Policy and Communications, Age Scotlandi both believed there was a place for online pharmacy, but that at the moment the best way to deliver the services expected was within communities where discussions and review could take place. Claire Fernie, Public Partner Volunteer, NHS Fifei raised the issue of data sharing when using online services.
We are of the view some of the face to face work carried out in communities could be undertaken digitally, akin to the engagements through the attend anywhere serviceiv.
The Cabinet Secretary for Health and Sport, Jeane Freeman, said—
"...we would have some concern if we were seeing significant evidence of online prescribing having an impact on community pharmacy."i
We believe the profession is perhaps underestimating the potentially disruptive effect of online pharmacies. We ask what consideration the Scottish Government has given to the potential threat of online pharmacies and how it can future proof the contract and associated regulations to protect the network.
Consumption
Waste

One of the underlying themes running throughout the inquiry was how to reduce waste associated with the consumption of medicines. It was proposed to us there were several ways in which waste could manifest itself—
Adherence to prescriptions - patients failing to take medicines as prescribed, not taking them at all and by not taking them in the manner described, failing to obtain the full clinical value of the medicine;
Medicines which have not been taken - medicines once prescribed and/ dispensed but are not taken cannot be brought back into the stock of medicines in the system;
Over-ordering by patients - patients ordering more than was required as part of repeat prescriptions;
Prescription of drugs which are ineffective - even when taken, not all medicine will work for everyone and taking ineffective drugs is as wasteful as not taking them at all; and
Errors and Harm
A number of respondents suggested there was insufficient information and understanding of how waste might arise and called for work to be commissioned to explore this area further.
Adherence
A Price Waterhouse Coopers reporti from December 2016 estimated 50% of patients do not take medicines as directed.
Several reasons were cited as to the causes and why lack of adherence was problematic—
It could result in drug resistant microorganisms emergingii;
Patients failing to take medicines as prescribed and denying themselves the clinical benefit;
Patients failing to take medicines but continuing to order them on repeat prescriptionsii;
Symptomatic of insufficient time with patientsii;
Suggested poor understanding on behalf of patients regarding their conditions and inadequate health literacyii; and
Side effects of medicines may cause patients to be reluctant to take themii.
Proposals to improve adherence
The Association of the British Pharmaceutical Industry (ABPI) saidi the NHS could learn from the support provided to patients during trials of medicines on how to take them which would improve adherence and Dr Sheuli Porkess, Executive Director of Research, Medical and Innovationii said adherence should be a public health priority. She further suggestedii the role of the pharmaceutical industry in improving adherence relied on feedback as to why patients were not taking medicines in order to develop products which suited patient needs better (such as one tablet in place of three).
Use of technology was also citediv as a way to support adherence, including dosette boxes, apps and text messages. Dr David Shackles of the Royal College of GPs told us technology can assist—
"We can use the repeat prescribing record to look back to see how well patients collect their medications, and to have conversations with our colleagues in community pharmacy, who alert us if medications are not being collected. There is a multidisciplinary team effort in that discussion. For example, community nursing colleagues might report back that there is a stockpile of medications in a patient’s home, which would alert us to the fact that they were not taking their medications as effectively as we might think."ii
Above all however, respondents overwhelmingly told us the most important aspect of improving adherence was in conversations with patients, both at the point of prescription and dispensing.
Matt Barclay, Director of Operations, Community Pharmacy Scotland said—
"It might be that patients themselves will make a decision, after an informed discussion, not to take a medicine, perhaps for reasons of harm or side effects or perhaps because the medicine is just not doing what the patient wanted it to do. That is at an individual patient or clinician-to-patient level."iv
Dr Scott Jamieson of the Royal College of GPs said "If a patient does not know why they are taking a medicine or what its intended benefit is, that is a failure of the system. When we have time to ensure that realistic conversations are being had with patients, and the positives of taking their medicine are being explained to them, they will be more likely to value the medicines and take them."ii He also said "When patients know why they are taking a medicine and value it, and they know the benefit of taking it, they are more willing to take it."ii
Dr David Shackles of the Royal College of GPsii spoke of the importance of discussions with patients to ensure they understood the medications and were sufficiently advised. He spoke of trust issues and working to ensure prescribers advice was taken. He noted issues of time for GPs to have such discussions with their patients.
Discussions with patients on adherence
Matt Barclay of Community Pharmacy Scotland said "In respect of long-term conditions and multi-morbidity, pharmacists—as the experts on medicines—obviously like to take a view and to have conversations with patients about what is prescribed for them, how they are taking those medicines and whether they are taking them at all", adding "In some instances, we know before the doctor knows when patients’ prescribing and pick-up patterns change. There are opportunities to ensure that the realistic medicine conversation...happens more frequently in community pharmacy."i
Matt Barclay alsoi said waste could be reduced through conversations with patients on adherence and getting benefit from medicines.
The Company Chemists Association said "Often the relationship between patient and pharmacist in the community gives a better insight into social issues surrounding medicines supply and adherence."iii
The Royal Pharmaceutical Society in Scotland (RPS) said the role of pharmacists in discussing medicines with patients was not suitably embedded within contractual arrangements.
Jonathan Burton, Chair, Scottish Pharmacy Board, RPSi, said patients were sometimes ashamed to admit to prescribers they had not taken a medicine as prescribed but would share that information with a pharmacist.
Community Pharmacy Scotlandv told us discussions with patients as to why medicines were being returned were not recorded, but patients were often very willing to explain why they were returning something.
Use of technology and packaging to support adherence
In August 2017, the Scottish Government published "Achieving excellence in pharmaceutical care: a strategy for Scotland"i which included a recognition of the need to transform delivery of services through use of technology. It stated—
"We are committed to this transformative programme of work which includes...technology enabled approaches to support people to better manage their medication.i"
The University of Strathclyde said "New sensor and monitoring technologies will enable key stakeholders from health care professionals through to the pharma producers to engage more effectively with patients and to understand compliance and adherence."iii
AstraZenecaiii proposed packaging could be better utilised to support adherence, providing an example of a 'smart inhaler' linked to an app to ensure the regime was adhered to.
The Association of the British Pharmaceutical Industry (ABPI)iii suggested indications could be printed on the packaging of medicines to support better management of conditions, leading us to question why this is not taking place. Dr Sheuli Porkess, Executive Director of Research, Medical and Innovation, ABPIvi told us of the regulation involved in packaging of medicines and suggested feedback from healthcare professionals would help in the design of better packaging. However, we question whether there is anything stopping manufacturers from seeking this directly. It was acknowledgedvii some companies were seeking this, but a comprehensive approach to sharing of information was called for.
We were interested in whether inclusion of the cost of medicines on packaging would improve adherence and the value placed on that by patients. Adam Osprey, Policy and Development Pharmacist, Community Pharmacy Scotlandvi suggested this may provoke unintended consequences by encouraging patients not to take expensive medicines to save the NHS money.
The Company Chemists Association suggested better use of technology could be made in order to support "patients with complex medication regimes, moving complicated medicines from hospital to community locations and addressing key medicine use issues."iii
Dr Scott Jamieson of the Royal College of GPs discussedvi software options to support those with long-term conditions to manage those better.
We were also interested in whether packaging could be designed to replicate the conditions required for the storage of some medicines, to address one of the reasons given that returned medicines cannot be reused. We note this approach was refutedvii by the ABPI.
Scottish Care lauded the eMar system, a process of barcoding, which it described as—
"significantly reduce medicines errors and also resulting unplanned hospital admissions. The average care home resident takes 7 medicines per day and this system not only makes it easy to track medicines thus reducing the risk of incorrect or missed doses, but can also create individualise and personalise alerts to medicines approaching their end date, or contraindications and so on. eMAR can also identify trends which may point to need for a medicines review. There should be resource available to roll out this system to all care homes in Scotland as standard practice."xii
Compliance aids
Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clyde said—
"Blister packs and compliance aids are sometimes viewed as a panacea, but I do not think that that is so. They are a solution to supporting patients to take their medicines as intended, but they bring risks. Some medicines in a compliance aid are not stable, and they can disempower the patient. Sometimes it would be better if the patient had a polypharmacy review to explore other strategies such as medicines administration records, known as MAR charts, or large-labels technology that could support better compliance. We ought to communicate better about that important area of risk"i
Campbell Shimmins of Community Pharmacy Scotlandi said compliance aids could play a part but concurred they were not a panacea and could increase, rather than decrease risks. He also advocated the role of reviews to reduce waste, before using compliance aids. The Royal Pharmaceutical Society in Scotlandiii told us of the time involved in compiling medicine trays, an aid to remind people at what time of day to take medicines, and suggested keeping these in their original packaging could help reduce waste. They also said the process made obsolete the manufacturers guarantee and, as not all medicines could be included, could increase the risk of a patient forgetting to take a medicine.
Environmental concerns
Jonathan Burton of the Royal Pharmaceutical Society in Scotlandi spoke on the issue of medicines ending up in landfill. He noted it was easy for people to return medicines to pharmacists and there was no need for it to end up in household waste. He suggested this should be part of wider waste disposal messaging, rather than just coming from health professionals. He felt emphasising the environmental messages might help with encouraging people to bring medicines back for disposal by pharmacists.
We were interested in how packaging could support proper disposal of items such as contact lenses to avoid these being flushed. Dr Sheuli Porkess of the ABPIi reiterated messages about regulation of packaging and said the industry was attempting to streamline packaging in so far as possible. Adam Ospreyi of Community Pharmacy Scotland surprised us with his suggestion this may prevent environmental messaging from being included.
Medicines which have not been taken
An issue raised in various written submissions was what happens to medicines which have been prescribed and the prescription has been presented to the pharmacy but then—
Not picked up; or
Picked up but not taken and returned
Medicines which are prescribed but not picked up
Respondents highlighted the issue of medicines which have been prescribed and requested from a pharmacy but have not been picked up. The perception of respondents was those medicines cannot then be assumed back into the stock of a pharmacy and are considered waste.
Campbell Shimmins of Community Pharmacy Scotlandi suggested less than "2 to 3%" of medicines requested will not be picked up. He said if medicines were still in the pharmacy and had been stored properly, they would be added back into the stock and not claimed for.
We were also interested in the reimbursement arrangement for this. Jonathan Burton of the Royal Pharmaceutical Society in Scotland said—
"...our systems are not technically proficient enough to account for every last tablet in the system that might or might not be collected by a patient. A certain amount of professional judgement and discretion and a sense of fair play are required in the pharmacy in relation to what is submitted for reimbursement and what is not. For example, we might dispense to a patient 95 tablets out of 100 because we do not have the other five in stock. If the patient never collects the other five and they just sit on our shelves, there is currently no way, technically, to ensure that we are paid for only 95 and not 100."i
He saidi pharmacies were expected to maintain a significant stock and were not recompensed for medicines which go out of date or were damaged while held in the pharmacy.
Jonathan Burtoni said used of technology, and in particular text messaging, allowed pharmacists to remind patients if they had not collected a prescription.
We recommend the Scottish Government investigate the issue of auditing systems in community pharmacy and whether this is resulting in over payments for pills which are not dispensed or collected.
Prescriptions which have been picked up but subsequently returned to the pharmacy unopened
It was suggested that medicines which have been picked up from the pharmacy in a prescription, but then returned without having been taken should not be considered waste. These are currently disposed of and cannot be re-dispensed.
Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clydei, Jonathan Burton of the Royal Pharmaceutical Society in Scotlandi and Dr Sheuli Porkess of the ABPIi said once a medicine had been dispensed to a care home or to a patient, and it had left the control of the pharmacy, the pharmacist could no longer be assured of its quality and so, even if returned unused and unopened, it could not be re-dispensed to another patient. Gail Caldwell later confirmediv this is not a blanket rule, but comes from regulation of pharmacists and it is they personally who must satisfy themselves as to the quality of a product. She also suggested the requirements of packaging for anti-counterfeit legislation would also make return difficult. The APBIv and the Royal Pharmaceutical Society in Scotlandvi informed us of World Health Organisation (WHO) guidance suggesting returned materials should "not be taken back as stock, but should be destroyed." ABPI also suggested for some low value medicines, the cost of ensuring their efficacy and quality would outweigh the cost of provision of a replacement.
Graeme Bryson, Director of Pharmacy, NHS Dumfries and Gallowayi, suggested there was less opportunity for waste in secondary care due to the closed nature of the system but acknowledged this did happen.
The main reason for the reluctance of pharmacists to re-dispense returned medicines related to storage and Gail Caldwell providediv us with extensive detail on testing, conditions of licence and packaging. Acknowledging there are legitimate concerns as to quality control, we were disappointed by the lack of innovative thinking presented to us which did not include suggestions as to how packaging and technology might address some of the issues highlighted by pharmacists for some if not all medicines.
An anomaly emerged on use of patients' own prescriptions in hospitals. Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clydei,told us that a patient's own medication continued to be used once they were admitted to hospitals, rather than writing a new prescription. She suggested it was safer for patients to use something they were familiar with to prevent something being taken twice.
This contradicts the principle of quality control cited as a reason not to use medicines which were returned to pharmacies un-used. Gail Caldwell suggested this is because it was not going to another patient and, through an assessment process, the quality and storage conditions of the medicine would be ascertained.
We consider the blanket opposition to the re-use of medicines to be wasteful. If the issue was quality and storage, it is not clear why it is deemed acceptable to give it to the person upon admission to hospital who could equally have wrongly stored it within their own home. This may undermine all the treatment being provided by the hospital, at a potentially much higher cost than providing new prescriptions.
The Cabinet Secretary for Health and Sport, Jeane Freeman was of the view "Boards should allow patients to manage their own medication, subject to a risk assessment. We are looking to ensure that that policy is in place across all boards, so that all patients are treated in the same way."i We sought the further detail as to how this would be achieved by the Cabinet Secretary for Health and Sport on this issue in correspondencexii. We also sought detail of analysis of where waste is occurring and why.
We urge the Scottish Government to work with the pharmaceutical industry to develop means of presenting medication for return unused which provides a guarantee for pharmacists and clinicians at to the maintenance of quality of that product.
Medicines which are dispensed but not taken
Dr Sheuli Porkess, Executive Director of Research, Medical and Innovation, ABPIi was keen to stress the need for feedback to the pharmaceutical industry on reasons for patients not taking medicines, which could be anything from packaging related issues to side effects. She noted pharmaceutical companies' interest in ensuring medicines were taken properly, as she believed doctors would not prescribe medicines which had no effect, and said industry wanted to be part of the solution. She acknowledgedii some companies were surveying patients to ascertain reasons for non-adherence in the absence of a comprehensive approach to collection of this information.
Over ordering by patients
Community Pharmacy Scotlandi suggested systems should be in place to identify patients who consistently over ordered medicines. NHS Grampian Area Drug and Therapeutics Committee (ADTC)i noted over ordering through repeat prescriptions could be done by patients themselves, but also where other parties, such as care homes, were ordering on behalf of the patient.
The issue of managed repeats was raised with us, but both Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clydeiii, and Campbell Shimmins, Community Pharmacy Scotlandiii, suggested this had been proven not to be a huge source of waste in of itself.
Claire Fernie, Public Partner Volunteer, NHS Fifeiii said "There are as many reasons for patients over-ordering as there are patients" but suggested fear of shortages and repeat prescriptions as two potential reasons for stockpiling. Adam Stachura, Head of Policy and Communications, Age Scotlandiii, suggested better systems of review would help with people ordering repeat prescriptions they no longer needed. He also noted a danger associated with people retaining and potentially taking medicines which were out of date.
Dr David Shackles of the Royal College of GPsiii suggested multidisciplinary teams may keep one another informed of issues such as stockpiling within a patient's home through informal channels.
Prescribing ineffective medicines
Many of the representations made to us in the course of our inquiry have focused on the need for evidence based prescribing and on the efficacy of drugs taken. Respondents and witnesses have told us taking ineffective drugs is as wasteful as not taking prescriptions at all.
Linked to this was the need for meaningful reviews of patient prescriptions and in depth discussions on the impact on the patient of taking medicines on the patient. Such discussions are considered in detail in the SOCIAL PRESCRIBING section and the REVIEWS OF PRESCRIPTIONS section of this report.
Errors and harm
Where an error occurs on a prescription in favour of over provision, it was suggestedi pharmacists found it easier to dispense the incorrect amount than to seek correction from the prescriber. This results in the patient being provided with an over supply which they cannot use and cannot return. Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotlandii said errors on prescriptions were sometimes raised with clinical colleagues but it seems are not recorded.
The Cabinet Secretary for Health and Sport, Jeane Freeman, said—
"Eleven per cent of all hospital admissions are attributable to medication-related harm, and half of those are preventable, so the work [polypharmacy guidance] reduces harm and waste. Integral to that is a patient discussion on adherence."ii
We are very concerned by this, especially in light of comments from pharmacists about it being easier to dispense in error than to correct prescriptions.
We recommend the Scottish Government review the issue of medicine related harm as a matter of urgency to ensure the safety of patients and prevent their admission to hospital.
We also recommend the Scottish Government consider acting to prevent prescriptions containing errors from being fulfilled, accompanied by a review of systems to ensure this is a sophisticated system based on more formal processes than hand written notes.
Research into the causes of waste
Several respondents suggested there was simply insufficient information and understanding of how waste might arise and called for work to be commissioned to explore this area further.
There was also an issue of how waste is measured. Campbell Shimmins of Community Pharmacy Scotlandi and Claire Fernie, Public Partner Volunteer, NHS Fifei told the Committee wasted medicines were recorded by weight, rather than by the value of the item. He also saidi there was no formal key performance indicator for waste in community pharmacy as there is in secondary care.
Disposal of medicines
Claire Fernie, Public Partner Volunteer, NHS Fife spoke of the experience in Fife of promoting the return of unused medicines to pharmacies, suggesting it was improving. She said—
"If someone has been prescribed medicine that they do not want or need, it becomes waste as soon as it goes out the pharmacy door. The majority of patients are not aware of that. We are making moves to get that information into the public domain through leaflets and poster campaigns in hospitals and GP surgeries, but we are not there yet."i
This raises the question for us of how a patient is to know they have been given something they do not need? The prescription by this point will have been through two qualified medical professionals and it would be hard for a patient to insist on giving it back to the pharmacy on the basis they do not think they need it.
We consider GPs and all other prescribers have a duty to explain the risks and benefits of taking medicines when writing prescriptions. We do not consider it is acceptable to cite a lack of time as an excuse for not providing this.
Similarly, we do not accept the absence of lines of communication between those engaging with patients taking medicines. All involved have, in our opinion, a duty to ensure proper communication occurs and is recorded. If this is required to be written into contracts then it must be done as a matter of urgency.
Manufacturers should be encouraged to consider packaging which both aids consumption and enables returned medicines when in date to be reused.
Having considered the arguments for putting the cost of medicines on packaging we are not convinced this would be beneficial.
We recommend the e-Mar system or an equivalent is introduced into all care homes and ask the Scottish Government how this could be mandated.
We recommend the Scottish Government improve awareness of the best way to dispose of medicines, such as the public awareness raising campaign in NHS Fife.
Waste - conclusions
The primary issue raised with us on the consumption of medicines was the potential for waste at this point in the process.
Lack of adherence to prescriptions was a cause of waste, and we heard a variety of proposals for solutions including patient support akin to that offered in clinical trials, use of technology and conversations with patients at the point of prescribing and dispensing. We also heard calls for public campaigns on adherence to medicines and improved health literacy.
While we understand the concerns of pharmacists over re-dispensing medicines which have left their control but been returned unopened, we consider there must be a solution, be that technological or packaging based. We urge the Scottish Government to work with industry to explore such options. We also look forward to hearing the outcome from of ensuring there is a uniform policy on the use of patients' own medicines in hospitals.
Feedback from patients and communication between all parties involved was cited as a key factor in reducing waste through people not taking medications once dispensed.
People cannot be allowed to languish on medication regimes which are ineffective for them and reviews are essential.
The safe disposal of medicines was also highlighted to us as important, not least from an environmental point of view. Initiatives such as those in NHS Fife to raise awareness on the safe disposal of medicines are to be encouraged.
Data and IT

The most prevalent theme throughout our inquiry was related to the use of data and the technology used to gather it. The University of Strathclyde said "Digital solutions can play a critical role in informing patients about all aspects of their treatments and provide more opportunities for them to engage with the appropriate experts if they have queries or concerns."i In every area and stage of the system of supply and demand for medicines in Scotland we considered, we heard significant improvements would be achieved through better collection of data and better technology to support that.
We have therefore included an additional chapter to our report summarising what we heard and allowing for system wide observations to be made. We are clear there needs to be consistent and uniform IT systems in operation across the NHS in Scotland.
The main issues arising were—
The sharing of medical records;
Need for a uniform system for medicines management across Scotland with accessibility by all areas of the health service;
The work of the Data Scoping Taskforce and the Digital Health and Care Strategy;
Preparedness for the medicines of the future;
Collection of outcome data; and
Digitisation of reviews of medicines.
Sharing of medical records
The case was made repeatedly for other medical professionals, but particularly community pharmacists to have access to patients' medical records in order to optimise their role. This will help ensure a smoother journey for patients through the various stages of the healthcare system and assist professionals while being in possession of all the facts. We heard about all of the following benefits—
Help to reduce polypharmacyi;
Monitor repeat prescribingi;
Assist with a reduction in wastagei;
Ease transfers between secondary and primary carei;
Support those with long-term conditionsi;
Uphold safety and provide a second set of eyes to spot errorsvi;
Help identify those at risk of non-adherence to prescriptionsi;
Provide an early alert and resolution to errorsi (which could help reduce unnecessary hospital admissionsi);
Expedite patient medicine reconciliation when someone moves from one part of the health service to anotheri - both Community Pharmacy Scotlandi and the Royal Pharmaceutical Society in Scotland called for discharge information to be shared with pharmacy;
Prevent valuable information on outcomes being lostvi;
Progress solutions for patients quickly within community pharmacy settingsvi;
Would allow pharmacists to work across multiple sitesi;
Give out-of-hours doctors the ability to access recordsvi;
Meet the expectations of patients, who would assume their doctor both within and outwith hospital, and subsequently their pharmacist could access recordsvi; and
Stop patients having to repeatedly tell their storyvi.
The NHS Scotland Directors of Pharmacyxviii called for investment in linking up data to be fully accessible to all professionals who needed it and suggested this should be linked to the national digital platform and owned by the patient.
We heard from both health boards and pharmacy that the absence of a joined up system between community pharmacy and the NHS "constrains clinical and cost effectiveness"i and impairs the ability of pharmacists to contribute their knowledge and intelligence gathered into patient records. These remain siloed at present where they exist.
We heard some health boards had already provided community pharmacists with access to records, in particular NHS Greater Glasgow and Clydevi said 63% of pharmacists in the board area had access.
We welcome the Scottish Government's swift move to optimise use of all parts of the system providing health care in Scotland in response to the COVID-19 pandemic. While welcome, these actions have highlighted the necessity for all parts of the health care system in Scotland to be operating based on joined up information and the need for appropriate parts of medical records to be make available to those who require them at the earliest opportunity.
It is clear to us a comprehensive system of care should include information sharing across all parts of the system and everybody providing patients with medical care and advice should have the requisite details of the patient in order to make evidence-based judgements. This applies equally to all.
Uniform IT and data systems
The need for uniform and comprehensive systems to collect and use data across Scotland was present strongly to us.
It will help—
Eradicate duplication of processes;
Reduced waste;
Accelerate communication across the health system;
Reduce errors and improve safety monitoring;
Support efficient use of old and new medicinesi;
Improve pricing and help secure better deals for the NHS;
Allow for the transfer of care from a secondary to a primary setting;
Optimise use of personalised medicines;
Improve economic outcomesi; and
Provide a basis on which to invest or disinvest in medicines.
Examples were abundant throughout our inquiry of efficient solutions which should be progressing but are not due to multiple IT systems in operation which cannot communicate easily.
There were calls for a uniform system across the NHS which enabled medical professionals at all levels to access the same information on the same system. We heardiii NHS Education for Scotland (NES) was currently working on the shared digital platform to link health and social care information, which we were told "cannot come soon enough".
We note the ambition in the "Achieving excellence in pharmaceutical care: a strategy for Scotland"—
"Our focus is on ensuring safe, effective and person-centred pharmaceutical care, and safer use of medicines is a core component of health and social care services in all settings. These settings include care homes and those requiring more care at home. We need to understand the enablers to deliver this which includes identifying the clinical capability and capacity required within the pharmacy workforce, the resources needed to improve IT system interoperability and leverage digital technologies and data and modernising our planning and delivery systems for securing NHS pharmaceutical care services."iv
Data Scoping Taskforce and the Digital Health and Care Strategy
The creation of a Data Scoping Taskforce was a recommendationi of the Review of Access to Medicines. Its purpose was to—
"...determine the digital capabilities required to utilise real-world health data to support the assessment and introduction of new medicines, together with ensuring the on-going safe, effective use of established medicines".
It made five key recommendations in its September 2018 reportii—
"Capture medicines use for patients in all clinical settings
Include medicine indication in all prescribing systems
Create a national laboratory data resource
Improve recording of patient outcomes
Create a Scottish Medicines Intelligence Unit"ii
Stakeholders were supportive of the recommendations of the report and called for action in several areas to fulfil them—
Build on use of the Chemotherapy Electronic Prescribing and Administration Systemiv;
Invest further in data infrastructuresiv;
Monitoring of prescribed treatment regimensiv;
Tracking uptake of new treatmentsiv;
Collecting information and examining the variation across boardsiv and across conditionsiv; and
Collecting information on why medicines are prescribediv.
In January 2020, the Cabinet Secretary for Health and Sport, Jeane Freeman, toldxi us the Scottish Government were considering a number of the recommendations as part of the implementation of the Health and Care Digital Strategy. That strategy was established in 2018 to deliver a National Digital Platform to provide the infrastructure, products and services to allow health and care technology to be delivered, managed and consumed in Scotland.
Scotland's Digital Health and Care Strategy
The Digital Health and Care Strategy also states the platform will allow for—
"Information capture and access at point of contact – providing up-to-date high quality and timely role based, secure access to multiple specialist health and care information and knowledge sources, which is essential and fundamental to enabling excellent care, supporting staff, empowering citizens and enabling self-care."i
In May 2019, we received an updateii on development of the National Digital Platform (NDP) which included—
Live testing of the platform was about to begin in NHS Greater Glasgow and Clyde and NHS Forth Valley in May 2019. Although, not all access would be read/write access;
Only clinicians would have access in the pilots; and
The national digital platform will communicate with "multiple historical systems".
As discussed in the SHARING OF MEDICAL RECORDS section of this report, we agree with the previous Cabinet Secretary for Health and Sport that interoperability across health and social care, including the community care sector, hospices and prisons, is absolutely essential and fundamental"iii and that all health professionals, be they working for the NHS or otherwise, who require access to records should have it and this must include involvement at the testing phase. We were disappointed to learn only clinicians were involved in pilots.
We are in no doubt the piecemeal approach to procurement of IT systems, such as has been the case with HEPMA, on a board by board basis across Scotland, is unhelpful and each board must be required to operate from the same systems.
Data Ownership
The Committee's view on who owns the patients' data has been clear and consistent through a number of recent inquiries; patient data belongs to the patient.
Technology for the medicines of the future
Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government, emphasisedi the need for "appropriate IT and data" to "cope" with the medicines of the future. Alison Culpan of the Association of the British Pharmaceutical Industry (ABPI)i and the University of Strathclydeiii agreed, with the latter noting achieving such systems of data collection was too much for one company or group to achieve alone and that Government support and incentives would be required.
We are of the view it is important to ensure the IT solutions proposed are fit for the revolutionised healthcare delivery that is coming and coming quickly.
Outcomes
The importance of collecting data on patient outcomes has been emphasised throughout this report.
Many have called for outcomes data from medicines to be collected to support evidence based prescribing and to eradicate waste by ensuring only the most effective drugs were used for patients in the first instance. The differences between outcomes achieved in clinical trials and those experienced in 'real-world' scenarios is important beyond being able to achieve innovative and cost effective pricing.
Cost effectiveness cannot be properly assessed without information on outcomes. This includes an understanding of the impact on patients.
The pharmaceutical industry rely on outcomes data to improve products and innovation in the Government's pricing arrangements similarly leant on such data.
Outcomes data for research, development, purchasing and procurement - Cancer Medicines Outcome Programme
The programme is a collaboration between NHS Greater Glasgow and Clyde and the University of Strathclyde to assess the impact on cancer patients of medicines in real-world settings and was being considered across different populations around the countryi. We understandii the scalability of the project across other clinical areas was being considered.
We would welcome further details on the timescales for this.
The Cabinet Secretary for Health and Sport, Jeane Freemaniii, also told us of other programmes to collect data based on patient experiences with cancer medications such as the Systematic Anti-Cancer Therapy (SACT) through ChemoCare system. On SACT she added a common approach for consent would be taken forward in early 2020 and data from the scheme was expected to be nationally available next year and be integrated with the New Scottish Cancer Registry and Intelligence Service.
We were pleased to learn an internal working group "to identify and prioritise a programme of work specifically in relation to improving data collection on medicines’ uses and outcomes, that will complement and support the overall objectives of the Health and Care Digital Strategy"iii has been established.
We are pleased to hear of the Cancer Medicines Outcome Programme and suggest this model should be rolled out across the NHS and for other ailments. The pharmaceutical industry should be involved in this work.
We ask the Scottish Government to provide details of the remit and work programme for the working group, including timescales for delivery and how it intends to bring together all the various projects taking place on data collection.
We also ask for detail on how the work of the internal working group on medicines will link with work on the National Digital Platform and the Health and Social Care Digital Strategy.
Prescribing
Throughout this inquiry we were surprised to learn how little evidence gathering of outcomes and effectiveness takes place. Yet the importance of data to support prescription decision making was emphasised to us from many parties.
The Royal College of GPs, who support annual awards with a focus on research and innovation in primary care, spoke about databases which included information linking interventions and outcomes, but noted the information and research in this area was not systematic or comprehensive. It was generally undertaken when investment was available with a view to publication.
The Royal College of Psychiatrists also saidi there needed to be better monitoring of deviation from standard models of care, and while acknowledging such activity was inevitable, saying a better understanding of those who did not respond to standard models of care or common medicines was required. They emphasised i the need for data collection to be at no extra burden to the clinician.
Consumption
Matt Barclay of Community Pharmacy Scotland toldi us some data on medicine use, along with adverse events, are probably haphazardly captured throughout the system, but not in any routine way.
We believe information on how patients are using medicines once dispensed is essential and this should be collected in a comprehensive and routine fashion.
Data and IT - conclusions
We are not surprised by the prominence of data and IT over the course of our work on medicines as it has been a recurring theme in most of our inquiries throughout the last four years. In particular, one of the two central pillars of our 2018 inquiry into technology and innovation in health and social care was "use of technology to transform the way medicine is practiced and delivered to patients".i
What we await to hear is how that will be achieved comprehensively and uniformly across the NHS in Scotland.
We ask the Scottish Government to reflect on why the sharing of medical records through the Emergency Care Summary suddenly became possible when COVID-19 struck and to make arrangements to extend access to health records to all health professionals who require them to ensure health care provision is as clinically and cost effective as possible. We request a date when this will be achieved.
We ask the Scottish Government in its response to this report to include—
full detail, including timescales, of the "programme of work"ii to improve data collection on medicines use and outcomes in Scotland.
A progress update on delivering the Health and Care Digital Strategy, including timescales for implementation of the "national digital platform"iii and how the "programme of work"ii will integrate with this.
We recommend all those with a role in delivering health and social care in Scotland have appropriate access to the national digital platform and again seeks timescales for how rapidly such access will be granted.
We recommend the Scottish Government provide detail of its influence on areas of research which would be beneficial in developing functioning outcomes gathering systems and how it supports/persuades other organisations to do the same.
We recommend the Scottish Government establish a means of collecting information on medicine use by patients.
Glossary
Definition of terms
Community Health Index number
"The Community Health Index (CHI) is a population register, which is used in Scotland for health care purposes. The CHI number uniquely identifies a person on the index"i.
End-of-Life Medicine
"A medicine used to treat a condition at a stage that usually leads to death within 3 years with currently available treatments."ii
General Medical Services Contract (GMS)
The General Medical Services Contract is negotiated between the Scottish Government and the British Medical Association and sets out what will be delivered by general practitioners (who are self employed) in Scotland.
National Therapeutic Indicators
"National Therapeutic Indicators (NTIs) and Additional Prescribing Measures (APMs) use prescription data to provide a measure of prescribing activity in specified therapeutic areas and a comparison across the GP practices in Scotland, Health Boards within Scotland and GP practices within these Health Boards. These are then made available to Health Boards, Health and Social Care Partnerships, GP practice clusters and GP practices for use in quality improvement initiatives and medicines management work. The relevant NTIs and APMs are included in the national prescribing strategy documents for Polypharmacy, Diabetes, Respiratory Medicine and Chronic Pain. They complement the national Realistic Medicine agenda and the Scottish Patient Safety Programme for Primary Care."iii
Orphan Medicine
"A medicine with European Medicines Agency (EMA) designated orphan status (i.e. conditions affecting fewer than 2,500 people in a population of 5 million) or a medicine to treat an equivalent size of populations irrespective of whether it has designated orphan status."ii
Patient Access Schemes
"Companies can submit Patient Access Schemes (PAS) to improve the cost effectiveness of a medicine. The Patient Access Scheme Assessment Group (PASAG) reviews and advises NHSScotland on the feasibility of proposed schemes for implementation. It operates separately from SMC to maintain the integrity of the assessment process."v
Patient and Clinician Engagement (PACE) process
"For medicines used to treat end of life and/or rare conditions, the Scottish Medicines Consortium (SMC) offers the submitting company the opportunity to request a Patient and Clinician Engagement (PACE) meeting which gives patient groups and clinicians a stronger voice in SMC decision making."v
Peer Approved Clinical System (PACS) Tier Two
"Clinicians (on behalf of their patients) can ask a Peer Approved Clinical System (PACS) Tier Two Panel within their NHS board whether they can access a medicine that has not been recommended by the Scottish Medicines Consortium and is therefore not routinely available in the NHS in Scotland. New Scottish Government guidance has now been issued which means that, in the event where a requesting clinician and patient feel they have grounds for a review of the local decision, the clinicians (on behalf of patients) are now able to ask for the decision to be referred to the National Review Panel. This replaces each NHS board’s local appeal process."vii
Pharmaceutical Pricing Regulation Scheme (PPRS)
"This voluntary scheme has been in place for over 50 years and helps to provide the pharmaceutical industry and the Government with a stable environment to introduce medicines into the NHS, making sure patients can get access to the newest, most effective treatments available, while helping to sustain a thriving UK pharmaceutical industry. A voluntary scheme has been negotiated every five years, with the next scheme due to start in January 2019. The current PPRS – negotiated in 2014 at a time of austerity in the UK – sought to cap the growth of NHS spend on branded medicines at an agreed rate, with any overspend paid back to the NHS. This was to provide stability of spend to the NHS and offer an incentive to use new medicines at no extra cost."viii
Pharmacotherapy
The new General Medical Services contract includes provision for pharmacists to be located within GP surgeries, known as pharmacotherapy.
Polypharmacy
"...the concurrent use of multiple medications by one individual."ix
Quality Adjusted Life Year (QALY)
"The health economics tool used to measure the benefit of a medicine is the quality-adjusted life year (QALY). This takes into account how a treatment affects a patient‘s quantity of life (how long they live for) and the quality of life (the quality of their remaining years of life). These factors are then combined into a single measure that puts a figure on the health benefits for a medicine. The resulting QALY can then be used to benchmark the benefits each medicine is likely to offer. Then, to consider the cost effectiveness of the medicine, the QALY is combined with the cost of the medicine to produce a ratio called the cost per QALY."x
Quality and Outcomes Framework (QOF)
Audit Scotland describe this as-
"...a voluntary incentive scheme for GPs which uses financial incentives to encourage high quality care. The QOF has had considerable influence on the way GPs work, including their prescribing. For example, it includes targets for managing particular conditions, such as hypertension, which have an effect on prescribing."xi
Repurposing
“Repurposing” is a concept used to describe a new use for an existing medicine which is already licensed for another treatment indication(s). The new use will usually be supported by some level of clinical data, and this may or may not, be planned to be submitted to a competent regulatory authority for licensing. In cases where the data is submitted to a regulator and is approved, the medicine will be granted a licence extension for that use as a new treatment indication."xii
Scottish Drug Tariff
"The Drug Tariff is published on behalf of Scottish Ministers. It sets out the rates payable for the provision of pharmaceutical services, the way in which reimbursement is calculated for drugs (both generic and proprietary products) and appliances supplied and it lists those appliances which are approved for supply."xiii
The Scottish Drug Tariffxiv is published by ISD Scotland.
Scottish Intercollegiate Guidelines Network (SIGN)
"The Scottish Intercollegiate Guidelines Network (SIGN) aims to improve the quality of healthcare for patients in Scotland by reducing variation in practice and outcomes through the development of national clinical guidelines. These guidelines provide evidence-based recommendations on the management of common conditions, including the use of medicines."xv
Ultra-Orphan Medicine
"To be considered as an ultra-orphan medicine all criteria listed should be met—
the condition* has a prevalence of 1 in 50,000 or less in Scotland,
the medicine has an EMA orphan designation for the condition and this is maintained at time of marketing authorisation,
the condition is chronic and severely disabling, and
the condition requires highly specialised management.
* SMC uses the description of the condition within the European Medicines Agency’s (EMA) Orphan Maintenance Assessment Report (OMAR) as a reference (or the description within the original orphan designation if the OMAR is not available).
Submissions for medicines that are validated as ultra-orphan according to this definition will be assessed by SMC and will then be available to prescribers for a period of up to three years while further clinical effectiveness data are gathered. After this period the company will be asked to provide an updated submission for reassessment and SMC will make a decision on routine use of the medicine in NHSScotland."v
Voluntary Patient Access Scheme (VPAS)
"VPAS ensures the annual spend on branded medicines can increase by a maximum of two percent every year. Anything over this increase is repaid by industry to the NHS."xvii
Acronyms
| Acronym | Meaning |
|---|---|
| ABPI | Association of the British Pharmaceutical Industry |
| CMOP | Cancer Medicines Outcome Programme |
| GMS contract | General Medical Services contract |
| HEPMA | Hospital Electronic Prescribing and Administration |
| HTA | Health Technology Assessment |
| IPTR | Individual Patient Treatment Request |
| NICE | National Institute for Health and Care Excellence |
| MHRA | Medicines and Healthcare products Regulatory Agency |
| MCR | Medicines Care Review |
| OT | Occupational Therapist |
| PACS | Peer Approved Clinical System |
| PACS T2 | Peer Approved Clinical System Tier 2 |
| PAS | Patient Access Schemes |
| PASAG | Patient Access Scheme Assessment Group |
| PPRS | Pharmaceutical Pricing Regulation Scheme |
| QALY | Quality Adjusted Lift Year |
| QOF | Quality and Outcomes Framework |
| SNF | Single National Formulary |
| SMC | Scottish Medicines Consortium |
| STAMP | European Commission Expert Group on Safe and Timely Access to Medicines for Patients (STAMP) |
| VPAS | Voluntary Patient Access Scheme |
Annexe A - Minutes of Meetings
18th Meeting, 2020 (Session 5) Tuesday 23 June 2020
7. Supply and demand for medicines inquiry (in private): The Committee considered and agreed a draft report.
17th Meeting, 2020 (Session 5) Wednesday 17 June 2020
4. Supply and demand for medicines inquiry (in private): The Committee considered a draft report. Various changes were agreed to, and the Committee agreed to consider a draft report, in private, at a future meeting.
16th Meeting, 2020 (Session 5) Tuesday 9 June 2020
3. Supply and demand for medicines (in private): The Committee considered a draft report. Various changes were agreed to, and the Committee agreed to consider a revised draft, in private, at a future meeting.
14th Meeting, 2020 (Session 5) Tuesday 2 June 2020
1. Supply and demand for medicines inquiry (in private): The Committee considered a draft report. Various changes were agreed to, and the Committee agreed to consider a revised draft, in private, at a future meeting.
6th Meeting, 2020 (Session 5) Tuesday 10 March 2020
1. The supply and demand for medicines inquiry: The Committee took evidence from— Jeane Freeman, Cabinet Secretary for Health and Sport, Rose Marie Parr, Chief Pharmaceutical Officer, Alison Strath, Principal Pharmaceutical Officer, Bryan Lamb, Head of Pharmacy Branch, and Alpana Mair, Head of Effective Prescribing and Therapeutics, Scottish Government.
3. The supply and demand for medicines inquiry (in private): The Committee considered the evidence heard earlier in the meeting.
4th Meeting, 2020 (Session 5) Tuesday 18 February 2020
1. The supply and demand for medicines inquiry: The Committee took evidence from—
Adam Stachura, Head of Policy and Communications, Age Scotland;
Claire Fernie, Public Partner Volunteer, NHS Fife;
and then from—
Dr David Shackles, Executive Officer for Interface and Out of Hours, Royal College of General Practitioners Scotland;
Adam Osprey, Policy and Development Pharmacist, Community Pharmacy Scotland;
Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland, Chair, Royal Pharmaceutical Society, Scottish Pharmacy Board;
Dr Sheuli Porkess, Executive Director of Research, Medical and Innovation, The Association of the British Pharmaceutical Industry;
Graeme Bryson, Director of Pharmacy, NHS Dumfries and Galloway.
3. The supply and demand for medicines inquiry (in private): The Committee considered the evidence heard earlier in the meeting.
3rd Meeting, 2020 (Session 5) Tuesday 4 February 2020
1. The supply and demand for medicines inquiry: The Committee took evidence from— Professor Angela Timoney, Director of Pharmacy, NHS Lothian;
Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clyde;
Campbell Shimmins, Community Pharmacist, Forth Valley, Community Pharmacy Scotland;
Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland.
2. The supply and demand for medicines inquiry (in private): The Committee considered the evidence heard earlier in the meeting.
2nd Meeting, 2020 (Session 5) Tuesday 28 January 2020
1. The supply and demand for medicines inquiry: The Committee took evidence from—
Dr Ewan Bell, National Clinical Lead, Area Drug and Therapeutics Committee Collaborative, Healthcare Improvement Scotland;
Matt Barclay, Director of Operations, Community Pharmacy Scotland;
Dr Scott Jamieson, Royal College of General Practitioners; David Coulson, Assistant Director of Pharmacy, NHS Tayside;
Eileen McKenna, Associate Director Professional Practice, Royal College of Nursing Scotland;
Jonathan Burton, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland, Chair, Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland;
Lewis Morrison, Chair of BMA Scotland, British Medical Association.
3. The supply and demand for medicines inquiry (in private): The Committee considered the evidence heard earlier in the meeting.
1st Meeting, 2020 (Session 5) Tuesday 21 January
1. The supply and demand for medicines inquiry: The Committee took evidence from—
Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government;
Elizabeth Woodeson, Director of Medicines and Pharmacy, Department of Health and Social Care, UK Government;
Jonathan Mogford, Director of Policy, Medicines and Healthcare products Regulatory Agency;
Alison Culpan, Scotland Director, The Association of the British Pharmaceutical Industry;
Warwick Smith, Director General, British Generic Manufacturers Association; Martin Sawer, Executive Director, Healthcare Distribution Association;
Dr Alan MacDonald, Chair, Scottish Medicines Consortium;
Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS National Services Scotland;
Matt Barclay, Director of Operations, Community Pharmacy Scotland;
Dr Brian Montgomery, Author of the Review of Access to New Medicines.
2. The supply and demand for medicines inquiry (in private): The Committee considered the evidence heard earlier in the meeting.
21st Meeting, 2019 (Session 5) Tuesday 24 September 2019
5. Medicines Inquiry (in private): The Committee considered and agreed its approach to the inquiry.
17th Meeting , 2019 (Session 5) Tuesday 25 June 2019
7. Medicines Inquiry (in private): The Committee considered its approach to the inquiry.
Annexe B
Written Submissions
The Committee received the following written submissions on the inquiry -
Supplementary Evidence
The Committee received supplementary evidence.
Correspondence
Letter to Jeane Freeman MSP, Cabinet Secretary for Health and Sport from Lewis Macdonald MSP, Convener of the Health and Sport Committee - 13 December 2019
Letter from Jeane Freeman MSP, Cabinet Secretary for Health and Sport to Lewis Macdonald MSP, Convener of the Health and Sport Committee - 13 January 2020
The Convener issued letters seeking further information following the evidence sessions on 21 January 2020:
Letter to Alison Culpan, Scotland Director, Association of the British Pharmaceutical Industry - 5 February 2020
Letter to Warwick Smith, Director General, British Generic Manufacturers Association - 5 February 2020
Letter to Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government - 5 February 2020
Letter to Matt Barclay, Director of Operations, Community Pharmacy Scotland - 5 February 2020
Letter to Elizabeth Woodeson, Director of Medicines and Pharmacy, Department of Health and Social Care, UK Government - 5 February 2020
Letter to Jonathan Mogford, Director of Policy, Medicines and Healthcare products Regulatory Agency (MHRA) - 5 February 2020
Letter to Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS National Services Scotland - 5 February 2020
Letter to Dr Alan MacDonald, Chair, Scottish Medicines Consortium - 5 February 2020
Letter to The Rt Hon Priti Patel MP, Secretary of State for the Home Department, Home Office - 6 February 2020
The Convener received the following responses to the above letters:
Letter from Matt Barclay, Director of Operations, Community Pharmacy Scotland - 20 February 2020
Letter from Lindsay McClure, Associate Director, Medicines Pricing and Supply, NHS National Services Scotland - 24 February 2020
Letter from Elizabeth Woodeson, Director of Medicines and Pharmacy, Department of Health and Social Care, UK Government - 26 February 2020
Letter from Alison Culpan, Scotland Director, Association of the British Pharmaceutical Industry - 26 February 2020
Letter from Rose Marie Parr, Chief Pharmaceutical Officer, Scottish Government - 26 February 2020
Letter from Dr Alan MacDonald, Chair, Scottish Medicines Consortium - 26 February 2020
Letter from Warwick Smith, Director General, British Generics Manufacturers Association - 26 February 2020
Letter from the Minister of State for Crime and Policing - 3 March 2020
Letter from Jonathan Mogford, Director of Policy, Medicines and Healthcare products Regulatory Agency (MHRA) - 4 March 2020
The Convener issued letters seeking further information following the evidence sessions on 28 January and 4 February 2020:
Letter to Matt Barclay, Director of Operations, Community Pharmacy Scotland - 17 February 2020
Letter to John Burns, Chief Executive, NHS Ayrshire and Arran - 17 February 2020
Letter to Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clyde - 17 February 2020
Letter to Professor Angela Timoney, Director of Pharmacy, NHS Lothian - 17 February 2020
Letter to David Coulson, Assistant Director of Pharmacy, NHS Tayside - 17 February 2020
Letter to Dr Scott Jamieson, Royal College of General Practitioners - 17 February 2020
Letter to Jonathan Burton, Chair of the Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland - 17 February 2020
The Convener received the following responses to the above letters:
Letter from Gail Caldwell, Director of Pharmacy, NHS Greater Glasgow and Clyde - 28 February 2020
Letter from David Coulson, Assistant Director of Pharmacy, NHS Tayside - 2 March 2020
Letter from Professor Angela Timoney, Director of Pharmacy, NHS Lothian - 2 March 2020
Letter from Dr Scott Jamieson, Royal College of General Practitioners - 2 March 2020
Letter from Matt Barclay, Director of Operations, Community Pharmacy Scotland - 3 March 2020
Letter from John Burns, Chief Executive, NHS Ayrshire and Arran - 3 March 2020
The Convener issued letters seeking further information following the evidence session on 18 February 2020:
Letter to Dr Sheuli Porkess, Executive Director of Research, Medical and Innovation, Association of the British Pharmaceutical Industry - 28 February 2020
Letter to Adam Stachura, Head of Policy and Communications, Age Scotland - 28 February 2020
Letter to Nicola Cotter, Head of GMC Scotland (General Medical Council) - 28 February 2020
Letter to Dr David Shackles, Executive Officer for Interface and Out of Hours, Royal College of General Practitioners - 28 February 2020
Letter to Jonathan Burton, Chair of the Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland - 28 February 2020
The Convener received the following responses to the above letters:
Letter from Nicola Cotter, Head of GMC Scotland (General Medical Council) - 4 March 2020
Letter from Dr David Shackles, Executive Officer for Interface and Out of Hours, Royal College of General Practitioners - 9 March 2020
Letter from Jonathan Burton, Chair of the Scottish Pharmacy Board, Royal Pharmaceutical Society in Scotland - 25 March 2020
Following the Cabinet Secretary for Health and Sport's evidence session on 10 March 2020, the Convener issued a letter seeking further information on a number of points:
Official reports
Tuesday 10 March 2020 - Evidence from the Cabinet Secretary for Health and Sport
Tuesday 18 February 2020 - Evidence from stakeholders
Tuesday 04 February 2020 - Evidence from stakeholders
Tuesday 28 January 2020 - Evidence from stakeholders
Tuesday 21 January 2020 - Evidence from stakeholders
Annexe C
| Pharmacists | Pharmacy Technicians | |
|---|---|---|
| Level one (core) |
|
|
| Level two (additional - advanced) |
|
|
| Level three (additional - specialist) |
|
|