Leaving the EU - Implications for Health and Social Care
This briefing summarises the potential implications of Brexit on health and social care services in Scotland. It focuses on issues such as workforce, reciprocal healthcare, new medicines, research and life sciences, the recognition of professional qualification and public health.
Executive Summary
Although the impact of leaving the EU on health and social care services in Scotland is difficult to forecast, there will be a number of implications. These will only become clear once the Withdrawal Agreement and the UK's future relationship with the European Union (EU) is finalised.
Leaving the EU could have implications for the regulation of health and social care in Scotland. If the UK leaves the single market and customs union there could be difficulties maintaining existing standards and procedures. The UK 's membership of the common regulatory bodies, such as the European Medicines Agency (EMA), would also come into question.
The health and social care sector in Scotland currently employs a number of workers from the European Economic Area. Prior to Brexit there were concerns around workforce vacancies and Brexit may amplify this through its potential to impact on the availability of overseas staff. Gaps in the data, both nationally and locally, make it difficult to estimate the extent to which the NHS and social care in Scotland rely on non-UK EU nationals. A survey of NHS boards and local authorities uncovered very few estimates of the number of non-UK EU nationals employed in Scotland. However, other data sources suggest that the NHS in Scotland may be less reliant on non-UK EU nationals than the NHS in other parts of the UK, but social care services may be more reliant than the NHS.
The future of Scottish patients' healthcare in other European countries is currently unclear. There are a number of elements of reciprocal healthcare and the free movement of healthcare which could be affected by Brexit. These include the European Health Insurance Card, healthcare for UK pensioners living in the EU and elective procedures.
Currently there is uncertainty surrounding the UK's membership of, or cooperation with, several EU agencies including the European Centre for Disease Prevention and Control (ECDP) and the European Atomic Energy Community (EURATOM) post Brexit. This could impact on the availability of medicines, healthcare techniques and technology.
Funding from the EU, and collaborative relationships, have had a significant impact on the quality of health research in the UK. As a global centre of research excellence, the UK has been one of the largest EU Member State beneficiaries of EU funding for health research since 2007.
There are also issues to be resolved surrounding good laboratory practice, legislation on blood, organs, tissues and cells, food compositional standards and labelling and data protection.
Brexit may present some opportunities in relation to the mutual recognition of qualifications and procurement policy in the NHS. Amendments to the Working Time Directive, although not universally welcomed, could also add to training opportunities for junior doctors.
Introduction
The UK is set to leave the EU in March 2019. There remains uncertainty about the impact of Brexit on health and social care in Scotland. EU Treaties identify the protection and improvement of human health as an area where the EU can carry out actions to support, coordinate or supplement the actions of Member States. As such, EU involvement in the delivery of Member State's health policies is limited. However, the EU is involved with health issues due to the single market and freedom of movement legislation. The UK’s vote to leave the EU could have significant indirect implications for health and social care in Scotland, not least because it has ushered in a period of economic and political uncertainty at a time when the health and social care system is already facing operational and financial pressures.
While the impact on health and social care services of leaving the EU is difficult to forecast, it is clear that a number of important issues will need to be resolved during negotiations. The way in which health policies are developed across the UK following Brexit is also a matter for further consideration.
The European Union (Withdrawal) Bill (the Withdrawal Bill) proposes that post-Brexit competences, which are currently carried out at an EU level, should be carried out by the UK Parliament and Government. The UK Government has suggested that repatriated powers should be retained at UK level to protect the UK's own single market and allow for the development of UK common frameworks. The Withdrawal Bill, proposes that once an agreement has been reached within the UK on the need for common frameworks, decisions will be made about what EU law should be kept.
On the 19 September 2017, Michael Russell, Minister for UK Negotiations on Scotland's Place in Europe, wrote to the Finance and Constitution Committee to outline why the Scottish Government could not recommend giving consent to the Withdrawal Bill as drafted. The letter set out a list of powers returning from the EU that he believes intersect with the devolution settlement in Scotland. Which, under the proposals, would become reserved matters. The Minister's letter stated that the Scottish Government believes that powers in devolved areas, which return from the EU, should be repatriated to the Scottish Parliament.
Context
There are several overarching themes in discussions on the future of Scottish and UK health and social care policy post-Brexit.
Regulatory Mechanism
Brexit is likely to affect regulatory mechanisms. A number of current healthcare initiatives and related legislation which originates from the EU rely upon the mutual recognition of standards and procedures. If the UK leaves the single market and customs union maintaining these standards and procedures may be difficult. The UK's membership of the common regulatory bodies would also come into question.
Arbitration
There has also been a concern surrounding the lack of a regulatory mechanism for resolving disputes. In August 2017, the UK Government published a policy paper on Enforcement and dispute resolution - a future partnership paper 1. This outlined that the direct jurisdiction of the Court of Justice of the European Union (CJEU) in the UK will end at the point of the UK's withdrawal from the EU. Several of the European bodies that the UK hopes to continue working with, such as the EMA and EURATOM, rely upon the CJEU to settle any disputes that may arise.
On the 8 December 2017 the EU and UK announced that a joint report had been agreed. The joint report is seen as a summary of negotiations towards the withdrawal agreement. The joint report outlined that the CJEU will have an indirect influence and that the UK courts shall have "due regard" to relevant CJEU decisions issued after Brexit2.
Negotiations
Another theme is the resistance of the remaining EU countries (the EU 27) to the UK's apparent wish to choose which aspects of EU membership it would like to retain1. There has been concern expressed that this could lead to a precedent being set for other nations, which may decide to leave the EU or modify their terms of membership. The UK Government has repeatedly stated that, while it rejects the single market and the free movement of people ,it wishes to continue its relationship with several EU healthcare agencies. This may not be possible and the UK may have to rely on alternative methods, which will be discussed throughout this paper.
Human Rights
There are also a number of human rights and equality issues related to health and social care that may be affected by Brexit. However, these will not be explored in detail in this paper. As a member of the EU, equality and human rights in the UK are protected under various EU laws as well as the Charter of Fundamental Rights of the European Union. The Charter brings together the essential human rights of everyone living in the EU, but it is proposed this will no longer have effect that the UK after Brexit. Not only could this have effect on several aspects of healthcare for UK citizens, it could also have an impact on EU living citizens in the UKi.
Although the Charter only applies when EU law is at stake, it is important to note that it can have more impact on reserved UK legislation as EU law has primacy over national law: in effect overriding it. Acts of the Scottish Parliament or actions of the Scottish Government are outside of legislative competence if they are incompatible with EU law or the European Commission of Human Rights. The Scottish Human Rights Commission highlighted these concerns, commenting that
…an EU exit may represent the loss of potential for the fuller protection of social rights, or principles, contained in the Charter such as workers’ rights, access to social security and healthcare1.
No Deal
The final recurrent theme in the literature focuses on the possibility of a no deal Brexit and highlights the uncertainty this would bring, as negotiations would not be realised. The implications of no deal are largely uncertain. However, it is possible that links with the EU would be lost and cooperation in all health related areas would end.
It is possible that, in the event of unsuccessful negotiations, the UK Government and the EU may seek to agree a number of specific bilateral agreements. One of which could cover issues affecting cooperation in health policy.
Workforce
One of the significant possible impacts of Brexit on health and social care, relates to workforce. The UK and Scottish NHS currently employs a number of workers from the European Economic Area (EEA), which is made easier by free movement rules arising from EU membership. Free movement of workers is a fundamental principle of the EU. It entitles EU citizens to look for a job, work without a permit, and live in another EU country. EU citizens also have the same access to employment, working conditions and social and tax advantages as nationals of that country.
Free movement of workers impacts the economy as a whole and will be central to the negotiations on the UK’s future relationship with the EU27ii. Whilst immigration policy is largely outwith the Scottish Parliament's competence - as it is reserved under Schedule 5 of the Scotland Act 1998 - the impact of post Brexit immigration law may have an impact on staffing in Scotland’s public services, including the NHS.
Workforce shortages, already a pressure in the NHS, may be one of the main risks of Brexit in the field of health and social care, as changes to ‘free movement of workers’ may result in difficulty recruiting and retaining staff.
The UK Government has reached an agreement with the European Union on citizens’ rights in negotiations on the UK’s withdrawal from the EU. This will allow EU citizens and their families already in the UK, to remain and work here after 29 March 2019. It is expected that this will be extended to resident citizens of Norway, Iceland, Lichtenstein and Switzerland. The UK Government has stated that, as the rights of British and Irish citizens in each other’s country are rooted in the Ireland Act 1949, Irish citizens will not need to apply for settled status.
However, uncertainty remains around the potential impact on NHS and social care services. This is because the extent to which EU nationals currently in Scotland will take advantage of the ability to remain is, as yet, unclear . The Institute for Public Policy Research has stated:
We recommend that the Government makes a particularly generous citizenship offer to NHS workers. Without them, the NHS would collapse.1.
In addition, the likelihood of future EU nationals wishing to come and work in the NHS and social care will also be dependent on the UK's immigration policies post-Brexit, which are yet to be agreed. These concerns are supported by the Royal College of Nursing which has called on Government to ensure that “long-term migration policy meets the needs of the health and social care sector”2.
In order to assess the potential impact on the NHS and social care services, it is important to gauge the number of EU nationals currently employed. However, there is little data available on the nationality of NHS and social care workers in Scotland. England, in comparison, has relatively comprehensive data. This shows that over 62,000 people from non-UK EU countries work in the English NHS, amounting to 5.6% of all staff: almost 10% of doctors and 7% of nurses3. Ninety thousand non-UK EU nationals work in adult social care in England (7% of staff)4.
The Scottish Government has estimated that there are 12,000 non-UK EU nationals working in health and social care in Scotland (3% of the total health and social care workforce)5 and that 4% of nurses and midwives are non-UK EU nationals6. However, these estimates are based on the annual population survey and comprehensive figures on the number of non-UK EU nationals employed by Scottish health boards and local authorities are not centrally available.
In order to get a better idea of the numbers, SPICe undertook a survey of NHS boards and local authorities on the data they held on the number of non-UK EU nationals they employed. The responses to this are outlined in detail in Annexe Aiii and described in the following sections.
NHS Board Survey Responses
Of the 14 health boards contacted, 12 responded but just 2 were able to provide any information. This was given with the caveat that the numbers provided may not be comprehensive.
NHS Ayrshire and Arran advised that they had records for 74 staff members from a non-UK EU country, with 40 of these working in the medical department. This equates to approximately 0.7% of the total staff headcounti.
NHS Borders advised that their estimated number of non-UK EU nationals was 80 out of 3,170 staff. This would be in the region of 2.5% of their total staff headcount [1].
Local Authority Survey Responses
Of the 32 local authorities surveyed, 20 responded. Of these, 7 were able to provide some information. Some of these responses came with the caveat that the figures provided were estimates or that they were unlikely to be complete as information on nationality was provided on a voluntary basis.
Nevertheless, of these 7 responses, the proportion of employees classed as non-UK EU/EEA nationals ranged from 0.5% in Dumfries & Galloway to 6.9% in the City of Edinburgh.
| Local Authority | Proportion of staff who are non-UK EU nationals or from the EEA |
|---|---|
| Aberdeenshire | 2.3% (56/2440) |
| City of Edinburgh | 6.9% (157/2288) |
| Dumfries & Galloway | 0.5% (3/601) |
| East Ayrshire | 0.9% (6/678) |
| North Ayrshire | 0.7% (4/557)i |
| Perth & Kinross | 0.8% (6/738) |
| Scottish Borders | 0.6% (6/1012) |
It is important to note that these figures relate to those who are employed directly by the local authority but a significant proportion of social care is delivered by the third and independent sectors. Workers in this sector will not be reflected in the above figures.
However, the response from Perth & Kinross Council helpfully provided the results of a survey it had conducted with the independent social care sector in its area. This looked at numbers of non-UK EEA nationals employed by independent social care providers. It found, that of the independent care homes that responded, 10% of employees were non-UK EEA nationals. The corresponding figure in the independent care at home sector was 7.8%. Combining these figures produces an estimate of 9.5% of employees in these sectors being non- UK EEA nationals.
Other Workforce Data Sources
There are a number of other data sources which can help provide an indication of the prevalence of non-UK EU nationals working in the NHS.
For example, the General Medical Council (GMC) produces statistics on doctors recorded on its professional register. This includes data on doctors who obtained their Primary Medical Qualification (PMQ) in other parts of the EEA. This is not a completely reliable proxy for a doctor's nationality as UK nationals will qualify in other parts of the EEA, and non-UK EEA nationals will study in the UK. However, it can be used to give some idea of the proportion of non-UK EEA nationals working in medicine in the UK.
The most recent statistics for Scotland show that 5.9% of doctors (n=1,177) working in Scotland obtained their PMQ in a non-UK EEA country. This compares to 8.7% of doctors in Northern Ireland, 8.5% of doctors in England and 6.4% of doctors in Wales.
The following table shows a breakdown of where non-UK EEA graduates in Scotland gained their qualification.
| Country of PMQ | Number | % of Total |
|---|---|---|
| Ireland | 251 | 21.3% |
| Germany | 169 | 14.4% |
| Poland | 143 | 12.1% |
| Greece | 99 | 8.4% |
| Spain | 74 | 6.3% |
| Italy | 67 | 5.7% |
| Malta | 62 | 5.3% |
| Romania | 51 | 4.3% |
| Hungary | 48 | 4.1% |
| Netherlands | 48 | 4.1% |
| Czech Republic | 30 | 2.5% |
| Bulgaria | 26 | 2.2% |
| Slovakia | 16 | 1.4% |
| Latvia | 15 | 1.3% |
| Belgium | 11 | 0.9% |
| Croatia | 11 | 0.9% |
| Lithuania | 11 | 0.9% |
| Austria | 9 | 0.8% |
| Denmark | 7 | 0.6% |
| Iceland | 7 | 0.6% |
| Sweden | 5 | 0.4% |
| Portugal | 4 | 0.3% |
| Estonia | 3 | 0.3% |
| France | 3 | 0.3% |
| Slovenia | 3 | 0.3% |
| Switzerland | 3 | 0.3% |
| Norway | 1 | 0.1% |
As can be seen above, there are 251 Irish-qualified doctors practising in Scotland, so the details of the ‘common travel area’ between the UK and Ireland may be significant in helping to retain those staff.
Among Scottish doctors, 9.3% of specialists and 3.8% of GPs graduated in parts of the EEA outwith the UK. The following shows how this compares to other UK countries:
| Specialist register | GP register | |
|---|---|---|
| Scotland | 573 (9.3%) | 225 (3.8%) |
| England | 8116 (13.3%) | 2446 (5%) |
| Wales | 328 (10.5%) | 98 (3.9%) |
| N Ireland | 214 (11.6%) | 175 (10.2%) |
Some specialities in Scotland have a higher representation of non-UK EEA graduates, for example, surgery (13%) and pathology (12.7%). In addition, certain geographic areas of Scotland may be more reliant than others on non-UK EEA graduates. For example, Western Isles Integration Joint Board noted that of 12 consultants, only one is Scottish while 8 are from other EU countries and 3 are non-EU1
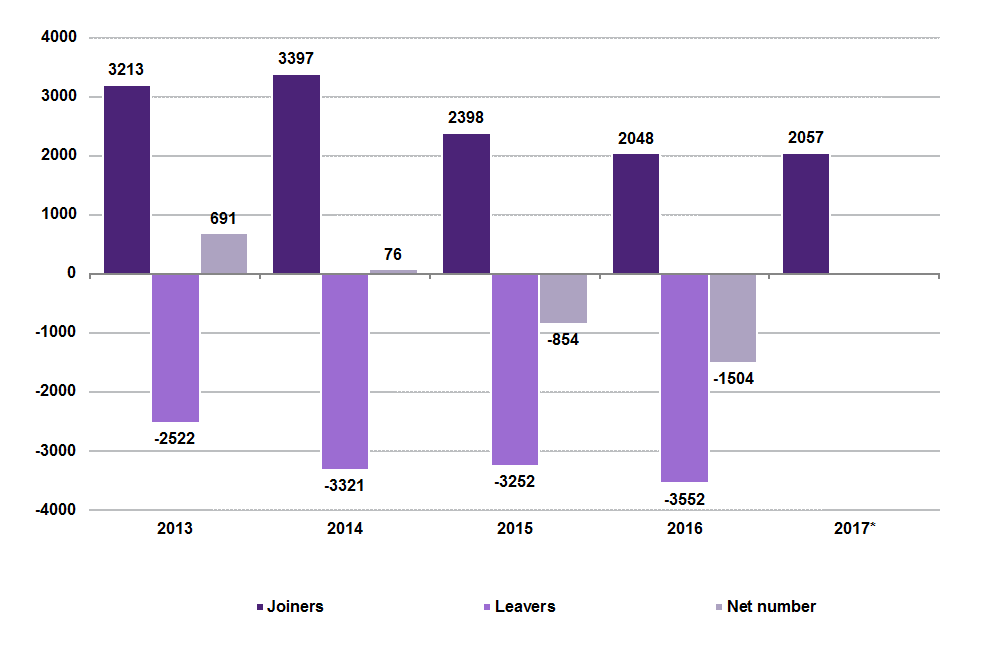
The current uncertainty over the outcome of Brexit may already be affecting recruitment and retention and there have been anecdotal accounts of staff leaving or planning to leave their jobs in the UK. The GMC records data on non-UK EEA graduates gaining and relinquishing their licence to practice in the UK. The following graph shows the number of 'leavers' and 'joiners' in Scotland from 2013 to 2017:
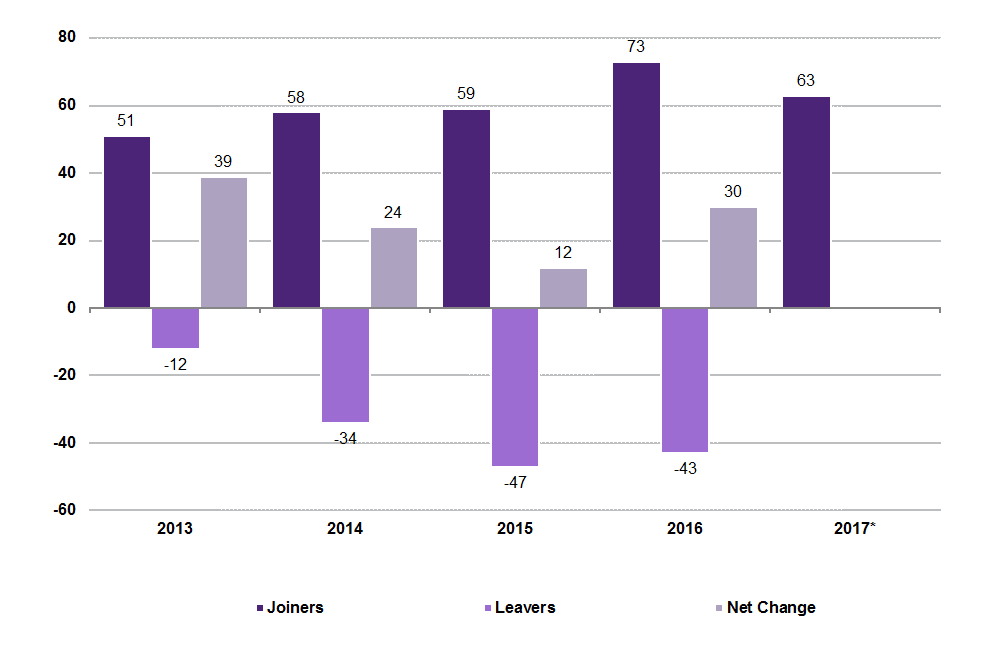
The number of non-UK EEA graduates in Scotland leaving is not yet available for 2017, but the number joining has decreased slightly over the last year. Whether this is a consequence of the EU referendum or not is unclear, but what we can say is that Scotland has maintained a net positive balance in the number of non-UK EEA graduate doctors while the UK as a whole has witnessed an increasing net deficit in the number joining/leaving. However, this trend predated the EU referendum and may in part be due to the introduction of English language requirements in 2014. This did not seem to have such a marked effect in Scotland.
There is also concern around nursing and midwifery staff, the largest staff group in the NHS. As of September, there were just under 690,000 nurses and midwives registered to work in the UK - over 1,600 less than the year before. The number of people from the EEA on the register has decreased by over 2,700 in a year from 38,992 (6.7%) to 36,259 (6.2%).
Figures published by the Nursing and Midwifery Council3 show that, while there are still nurses and midwives coming to the UK from the EU, newcomers in 2017 dropped by 89% as compared with the previous year. Although, like doctors, the recent introduction of a higher standard of language test for EU staff may have had an impact on the number of migrant EU nurses4. However, the NMC reports that there has also been a 67% increase in the number of EU nurses and midwives leaving the register in the 12 months up to September 2017 compared to the same period the year before. Unfortunately the NMC figures are not broken down into the constituent UK countries so it is difficult to gauge whether these trends apply to Scotland also.
However, the concern comes at a time when unions report that there are significant shortages in the number of nurses employed by the NHS. Figures from NHS Scotland5 show that, as of September 2017, 4.5% of nursing and midwifery posts were vacant, up from 4.2% in the last year and the equivalent of 2789.2 whole time equivalent posts. Of current vacancies, about 30% had been vacant for over 3 months (n=826.9 WTE). Amongst doctors, 7.7% of consultant posts were vacant on 30 September 2017, up from 7% in the previous year. More strikingly, 59% of these posts had been vacant for more than 6 months. There are also concerns about the ageing NHS workforce in Scotland and what this means for the future of NHS staffing6
Working Time Directive
In 2016/17, NHS Scotland employed almost 140,000 whole-time equivalent staff 1 and a further 20,000 people work in social work and social care - around 1 in 13 people in employment in Scotland2. Given the size of this workforce, the prevalence of part-time and shift-work, and the number of agency workers, EU employment legislation is highly relevant for many staff.
The Working Time Directive was introduced by the EU in 1993 and has been successfully implemented in the NHS since its inception. Broadly speaking, it limits the time staff can work to 48 hours per week and sets out the minimum daily and weekly rest breaks. These rules include time that is spent on call, and this cannot be opted out of collectively, it is up to the individual3
The Working Time Directive is currently implemented by the Working Time Regulations 1998 (as amended) and, under the terms of the Withdrawal Bill, the Directive will pass into UK Law. However, Brexit may present an opportunity to amend the current legislation to enable health care workers to work longer shifts.
The primary criticism of the Working Time Directive relates to junior doctors. Where previously long on call hours had been used to combine patient care and learning4, this has not been possible since the Directive was implemented. Following the changed to the working hours rules there has been an increase in the use of shift based systems.
Although organisations, such as the Royal Colleges, have said that the effect of the Working Time Directive has been mostly negative5, and that Brexit presents an opportunity to rectify this, Unison has expressed the view that workers’ rights may be diminished after Brexit6.
For the NHS, and social care post-Brexit, any future plans to change the working hours rules will need to recognise that a return to the old system may have a negative impact on patient care and on the doctors themselves. Longer working hours in general are now seen as less acceptable and many training practices have changed to reflect this view7.
Given that working hours are reflected in terms and conditions under the Agenda for Change8 and current staff contracts, any change to the terms of the Working Time Directive could result in a requirement for contract negotiations.
Elements of Reciprocal Healthcare/Free Movement of Healthcare
European Health Insurance Card
The European Health Insurance Card (EHIC) enables EU citizens to receive urgent or emergency healthcare, on the same basis as the local population, while travelling in EU/EEA member states.
Around 27 million UK citizens have an EHIC. Although, only approximately 1% of those holding a card make a claim each year, costing the UK £150 million1.
The issue of future healthcare arrangements, including the EHIC card, is an issue which will be discussed during the second phase of negotiations2. The Secretary of State for Exiting the European Union, David Davis, has stated that it has been agreed that access to the EHIC card will remain post Brexit3.
If the EHIC or a similar replacement system, is not agreed then UK citizens travelling within the EU may need to take out health and travel insurance. McMillan Cancer, in their written submission to the House of Commons Health Committee, stated that this would make travel within the EU for those with cancer difficult, as the cost would be prohibitive for them and indeed for any citizen travelling with pre-existing conditions4.
UK Pension Recipients
The EU system of reciprocal healthcare is tied to the system of benefits in general. If a benefit or pension entitlement is exportable to another EEA country, then healthcare entitlement automatically follows1. The entitlement to reciprocal healthcare depends on the idea of insurability, which is to say, that the arrangement is based on the idea that the costs of healthcare are borne by the country in which the individual is insured, and the country of treatment will be reimbursed by the insuring state1. This is known as the S1 scheme and is grounded in Regulation (EC) 883/2004.
The joint EU-UK document on the progress of negotiations has indicated that agreement has been reached on retaining S1 coverage for those already living in other countries on the day of the UK's exit3. An alternative option would be the negotiation of bilateral agreements which was the position before the UK's accession to the EU in 19724.
The House of Commons Health Committee heard evidence that Brexit could actually present an advantage in redressing the balance in terms of costs for reciprocal healthcare. In 2015, EU member states claimed £674 million in reimbursement costs from the UK, whereas the UK claimed £49.7 million in return5. It has been suggested that this is due to the 190,0006 UK pensioners in EEA countries signed up to the S1 arrangements who accounted for £500 million of the claims6.
Conversely, it has been suggested that, if UK pensioners living abroad were to return to the UK post Brexit, there would be increased pressure on health and social care services. Around 190,0006 British pensioners have chosen to live in another EU country under this scheme. If this population could no longer receive free healthcare in their country of residence they may return to the UK to be treated. This is projected to cost the NHS around £1billion a year9, twice what is being paid to the countries currently treating them. It is also likely to have a significant impact on resources as these 190,000 people would require 900 more hospital beds and 1,600 additional nurses9. These statistics refer to NHS England, but NHS Scotland is likely to face similar problems.
Some UK citizens living in EU states will still be entitled to some aspects of healthcare through the domestic legislation of the country in which they live11. However, these rights are unlikely to be universal, are hard to enforce, and will only relate to those people established in another EU country who are fully contributing to that country's benefits scheme through paying tax etc. Moreover, UK citizens who live in one EU country and work in another (which is reasonably common in central Europe) could be faced with serious challenges in receiving the care that they need11.
Elective Procedures
Directive 2011/24/EU76 and the council on patients’ rights in cross border health care are implemented in Scotland by the National Health Service (Cross-border Health Care)(Scotland) Regulations 2013, which was further amended in 2015 to allow for the recognition of medical prescriptions between member states1.
When a patient has travelled for the specific purpose of receiving treatment, the regulations allow for a reimbursement up to the level of costs which would have otherwise been borne by the home country. This provision only takes effect when the patient would have been entitled to the equivalent treatment at home. Such treatment needs prior authorisation from the home health board, which is able to refuse if the treatment can be provided in the home state within a medically justifiable time limit. This can lead to some discrepancy in application as it is up to a medical professional to decide what an appropriate waiting time is. This option is not frequently used and, consequently, has been less of a priority in the Brexit negations1.
New Medicines, Devices and Clinical Trials
New Medicines
A thriving pharmaceutical industry is important to the NHS. Not only does it allow patients quick access to new medicines, but it is estimated that life sciences contribute around £8.6 billion to public funds in the UK1.
The EU accounts for 25% of the world's sale of medicines and its regulator, the European Medicines Agency (EMA), is considered one of the most significant2. The UK accounts for just 3% of medicine sales globally.
The system for approval of medicines within the EU is two-fold. The EMA, as a centralised, co-ordinating body, makes an assessment for the European Commission (EC) about which medicines should be formally approved3. This centralised procedure prevents duplication of spending and work. It also makes the UK and other EU countries a priority for the introduction of new drugs. The Medicines and Healthcare products Regulatory Agency (MHRA) has its own procedures for licensing medicines, which are not covered through the centralised procedure of the EMA. Once a medicine is approved for use by one of these regulatory agencies, the Scottish Medicines Consortium (SMC) assesses how well the medicine works in relation to treatments currently used in the NHS in Scotland and whether they should be funded routinely by domestic health services4. The association of the British Pharmaceutical Industry notes how the EMA has:
not only greatly simplified the situation but also resulted in a system where medicines information such as the patient information leaflet are consistent across all EU member states, which is good for public health protection.5
The UK's continued membership of the EMA after Brexit is unclear. There have been several options outlined for the period after the UK leaves the EU. The first of these is retaining a single process that would work across both British and European legal jurisdictions6. In practice, this would be a process in which pharmaceutical companies could approach either the MHRA or the EMA with a new product, an assessment would be carried out and any recommendations would be made to both parties. However, this would rely on an agreement between the two parties that they would respect each other's decisions. The mutual trust required for this already exists to a certain extent as the EMA already outsources a third of its work to the MHRA7 and there is, albeit informally, a reliance on assessment reports which have been carried out elsewhere8.
Another option is that the MHRA could generally accept EMA judgements and approvals on new products, even if this agreement is not reciprocal. This could mean that the UK would stay part of the larger market and continue to get preferential access to drugs and treatments. It has also been proposed that the UK could use judgements from other international regulators such as the US Food and Drug Administration (FDA)9. Countries such as Singapore already accelerate the recognition of medicines that have been approved by other developed nations10. The UK could join with other countries, such as Australia or Japan, to create a large agency in its own right. However, this would take time and could lead to uncertainty in the interim.
If the UK were to agree to rely on assessments carried out by other agencies instead of carrying out its own assessments, there is scope for savings. The main disadvantage to using this sort of model is that it could damage Scotland's and the UK's pharmaceutical and research industries as work would be outsourced and, with it, resources and expertise. Conversely, if the UK decided to preserve its ability to make its own decisions and assessments, it could give the UK the opportunity to further develop its own regulatory system for drugs, to speed up the approval process, theoretically allowing quicker access to drugs and medicines. The Law Society of Scotland4 has also posed the question as to whether the role of the SMC could change to become a licensing authority in its own right, or if there could be an enhanced role for the MHRA in terms of technology assessment. An enhanced role for the SMC would require further devolution to Scotland, as the regulation of medicines is reserved to Westminster.
The Lancet has suggested that any separation between the MHRA and the EMA could prove very costly for taxpayers in both the UK and the EU. The reason for this is that domestically, there would be a delay in access to drugs and a need for investment in our own regulatory system. The EU would need to replace a third of their workforce and British expertise. In Switzerland and Canada, which have separate approval systems, medicines typically reach the market six months later than in the EU12.
Brexit also poses potentially serious questions around the availability of medicines in the UK. As it stands, the UK benefits from what is called "parallel trade". Parallel trade means that NHS buyers can procure medicines from EU countries where they are available at a lower price and, in turn, pass this saving on to the NHS. It has been estimated that this saves the NHS around £100 million a year13 so any loss of access to this scheme would result in a significant spending increase. These losses could be limited through post Brexit domestic legislation to prevent pharmaceutical companies using intellectual property rights to limit EU imports14. This would likely be incorporated into a future Trade Bill15.
Another key international regulatory body on which the UK is set to lose influence is the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. The ICH aims to harmonise development and registration of pharmaceuticals globally through standards on good clinical practice relating to the quality, safety and efficacy of pharmaceuticals. The UK is currently an influential member of the ICH through its membership of the EU16. However, this will not be the case after the UK leaves the EU. The MHRA is likely to reapply to become a member of the ICH as soon as it leaves the EU16. This would require an application for observer status before becoming a regulatory member which would take at least two years. During this time, the UK would be held to the ICH regulations but would not have an opportunity to influence them. If the UK gains full regulatory membership of the ICH, it would be able to exert its voice on behalf of the UK rather than having to represent the interests of Europe16.
On 23 January 2018, the EMA launched a survey to gather information from marketing authorisation holders of centrally authorised medicines who are located in the UK or who have quality control, batch release and/or import manufacturing sites, or a QPPV or pharmacovigilance system master file (PSMF), in the UK, on their plans to submit transfers, notifications or variations to their marketing authorisations in preparation for Brexit. The aim is to:
identify companies where there is a need for concerted action to address medicines supply concerns due to Brexit in order to protect human and animal health;
help EMA and the Commission plan resources in the areas where these submissions will be processed19.
Medical Devices
Most of the legal framework governing the UK's medical devices sector originates from the EU and is either directly applicable (through Regulations) or indirectly applicable through the UK Government implementing EU Directives. The framework is enshrined in the Medical Devices Regulations 2002 (as amended) which implements the following EU directives: Directive 90/385/EEC relating to active implantable medical devices; Directive 98/79/EC relating to in vitro diagnostic medical devices; and Directive 93/42/EEC relating to medical devices. Replacements for these directives were adopted by the European Parliament and Council in 2017 and will apply after a three and five year period respectively. The current EU system is being updated in stages which could potentially leave the EU and UK open to a regulatory divergence affecting both groups.
Medical devices are regulated in the EU using the CE marking scheme which certifies its compliance with EU law1. These marks of approval are given by notified bodies in the relevant member states, including several in the UK. Manufacturers apply for their device to be approved in a particular framework, effectively describing what the device's intended use is. When the required standard is achieved, the Notified Body informs the MHRA and the manufacturer is allowed to place a CE mark on the product1. This certifies that the product is safe and allows it to be sold anywhere in the EU. Currently, the national device regulators, such as the MRHA, focus primarily on the continued monitoring of the safety of devices marketed in their jurisdiction and in the sharing of this information with other member states1.
A potential implication for medical devices, post Brexit, is that many medical devices are currently imported from the EU4. The Withdrawal Bill notes that the UK will continue to permit the sale of CE items5 . This will avoid forcing companies to go through a separate regulation process which might put them off the UK market.
There are potential implications for the 94,000 people employed by medical technology companies in the UK6 as their employers may face incentives to leave the UK. There could also be implications for the UK economy as the British medical device technology industry is worth £17 billion or 6% of the global market7 .
Post-Brexit, the UK will no longer have an influence in shaping EU legislation, policy and regulatory procedures. As for the medicines sector, there would be scope for the UK to create its own system of certification as is recommended by the British Standards Institute8. Although this would require some spending, it would put the UK in a similar position to Australia9 and Switzerland in that it would negotiate for UK bodies to be able to sign off on EU compliant devices as well as having its own set of rules domestically.
Clinical Trials
Clinical trials could also be impacted by Brexit. The EU’s previous clinical trials directive, Directive 2001/20/EC, was criticised for being too bureaucratic1. However, the new set of regulations to be introduced in 2018 are widely seen as an improvement. In evidence to the Science and Technology Committee2 universities and science bodies commented that they favoured the UK implementing this new directive to avoid changing regulations mid trial. There is also a risk that, having separate regulations to other EU countries, would disadvantage NHS patients as they may lose access to trials that they previously had access to.
Research and Life Sciences
EU funding and the collaborative relationships which have developed have had a significant and positive impact on the quality of health research in the UK. As a global centre of research excellence, the UK has been one of the largest beneficiaries of EU funding for health research since 20071. However, while the UK falls behind similar economies in investing in research and development, it attracts substantially more funds than it contributes to EU research funding2.
One of the biggest sources of research funding in the UK is Horizon 2020, an EU programme that promotes research into topics as diverse as health and well-being, green transport, outer space and future technologies. Since 2014, it has contributed €420 million to health research in the UK3. EU direct funding accounts for 17% of research contracts, but accounts for almost three quarters of the growth in funding in the past decade2. In Scotland it has been suggested that 13% of all research funding derives from EU sources and, in 2014/15,Scotland's 19 higher education institutions secured £94 million in research grants from EU bodies, accounting for 9.4% of total funding5. Edinburgh University alone accounts for 30% of Scotland's 480 Horizon 2020 grants, making it the seventh largest higher education recipient of these funds worldwide6.
Non EU members can participate in Horizon 2020 but they are not eligible to be full members. The UK Government has said that it will guarantee money allocated under Horizon 2020 whilst the UK is still part of the EU7. However, it is not clear what will happen to funding after 2020.
The other advantages of membership, namely eligibility for future funding rounds and the opportunity for UK scientists and institutions to make joint bids that would put them at the forefront of international science and research, would be lost. It has been reported that UK health researchers are being “bumped off grant applications to the EU” due to the uncertainty surrounding the UK's future in Horizon 20208.
Post-Brexit, the UK will not have voting rights, although there may be opportunities to influence some decisions through committee work. Although some associate members receive more funding than they contribute9 this may be unlikely in the UK's case due to the political sensitivities surrounding Brexit. Switzerland, for example was denied access for some time to parts of Horizon 2020 as a result of its previous refusal to honour freedom of movement10.
The UK Government has highlighted its willingness to maintain and strengthen cooperation with EU partners in the realm of science and innovation through proposing plans for a new science and innovation agreement.
Concerns have also been raised about the long-term future of EU students and researchers currently studying/working in healthcare related fields in Scotland. Currently, EU students account for 15.9% of postgraduate research students, 11% of all staff, and 24.8% of research staff5.
In Scotland, life sciences, although representing a small sector of the Scottish economy, are high value and highly export orientated. They are key components of Scotland’s innovation, manufacturing and technology base. While Brexit poses a risk to future funding, there are some indications that leaving the EU could have a positive effective on the life sciences sector in Scotland. Due to the international orientation of the market, any decline in the value of Sterling has the potential to incentivise Scottish companies to export more globally. The recent £110 million investment in the GlaxoSmithKline plant in Grangemouth suggests a positive future, irrespective of the UK’s decision to leave the EU 12.
Mutual Recognition of Professional Qualifications
EU Directive 2005/36/EC (amended by Directive 2013/55/EU) allows for the recognition of professional qualifications across the EU with the aim of enabling free movement of professionals such as nurses, midwives, doctors, dentists and pharmacists.
It sets out the rules that allow for temporary mobility for workers wishing to establish themselves in another EU country, and for the automatic recognition of qualifications, as long as there are harmonised minimum training conditions in place (as there is in healthcare related jobs). The relevant sections of these regulations have now been incorporated into the Medical Act 1983. The professional regulation of doctors is reserved to Westminster, but the General Medical Council (GMC) operates within the legal and legislative structures of the different jurisdictions within the UK. It is expected that, as a result of Brexit, the UK Department of Health will review the Medical Act 1983 and, in consultation with devolved administrations, authorities and professional regulators, will decide whether or not to abolish, retain, or modify EU Law1.
Four possible outcomes have been proposed regarding the recognition of professional qualifications post-Brexit:
The UK remains in the single market and non-UK EEA qualified staff continue to have their qualifications recognised.
The UK leaves the single market and continues to recognise qualifications unless the UK Government repeals the relevant provisions from the Medical Act 1983.
The UK leaves the single market and the UK Government makes significant changes to the way non-UK EEA qualified medical staff are regulated.
The mutual recognition of all European professional qualifications ceases.2
Although there are significant benefits to high levels of mobility for medical professionals and the UK has undoubtedly benefited from the inward flow of dedicated professionals from the EU, concerns have been raised about the current EU directives in written evidence to the House of Commons Health Committee. The GMC sees this as a potential opportunity, as it believes current EU law has created a weakness in the system. As it stands, doctors who qualified overseas (non EEA) are subject to rigorous assessment of their knowledge and skills in order to test their competence2. Without EU oversight, there is the potential for these tests to apply to non-UK EEA nationals as well. The GMC further states that because of variability in training in EU countries, doctors coming to work in the UK may have gaps in their knowledge or skill sets2. A more stringent assessment would provide assurance to patients that such doctors meet the necessary standards of safety and good practice. The Nursing and Midwifery Council's expanded on this point by stating that currently there is a requirement to recognise a nurse or midwives' qualifications, even if they have not practised for a significant period of time2.
Future decisions on the the recognition of professional qualifications will be discussed in the second phase of negotiations6.
Public Health
Currently, cross border threats to public health are monitored by the European Centre for Disease Prevention and Control (ECDC) in Sweden. The ECDC runs systems for the surveillance and early detection of communicable diseases which facilitates prompt sharing of information and expertise when required. As it stands, only members of the EU and EEA countries are members.
Although public health protection is a devolved matter, it is still reliant upon international networks. There are several approaches that could be taken post-Brexit. Health Protection Scotland was established in 2005 to co-ordinate, strengthen and support activities aimed at protecting the Scottish population from infections and environmental hazards. It already links with partners in other parts of the UK and internationally to ensure that information is shared, as well as advising the Scottish Government of credible threats and strategic management.
It is currently unclear what mechanism might be put in place for sharing information and expertise on communicable diseases and cross border threats, post-Brexit. Norway, Lichtenstein and Iceland participate as EEA/EFTA countries in several EU agencies, including the ECDC, through agreements that specify financial contributions and roles. However, they are not full member states and do not, therefore, have voting rights or decision-making powers despite their financial contributions.
Another option is to create a UK wide agency to share information between countries. However this would still need a high level of collaboration with the ECDC. The ECDC works with the World Health Organisation's (WHO) Europe branch which the UK will remain a member of, but this only relates to certain diseases.
The ECDC also has memoranda of understanding with the US and Chinese disease control centres and with the WHO to facilitate sharing of information and expertise, so it is possible that any Scottish or UK equivalent centres could implement a similar arrangement.
Jeremy Hunt, Secretary of State for Health, does not believe that the UK will have to leave the ECDC. He said:
Obviously, we want to continue all aspects of co-operation with our partners and friends in the EU post-Brexit in order to reduce public health risks. It is incredibly unlikely that they will not want to do that, because it is as much in their interests as it is in ours1
Cross national approaches to public health are necessary for dealing with health threats and related issues that do not stop at country borders. They are also effective when dealing with large, multi-national corporations that individual countries can only have limited influence over. The EU has made environmental factors affecting public health a legislative priority2. For example, the EU issued a series of Directives that limited the sulphur content of both fuels and emissions from power plants and industrial sites3. These directives have been associated with an 80% fall in emissions Europe wide4. Another example relates to emissions from vehicle engines where the EU imposed engine standards that led to road traffic emissions being reduced by 63%5. The focus on environmental factors is exemplified by the EC action against the UK to enforce the Air Quality Directive65. EU directives also address water quality on several different levels. There are standards for both drinking water and bathing water, with the latter being enforced through the ‘Blue Flag’ system for beaches. Only 65% of British beaches achieved an excellent rating in the EU’s classification system, which is the third lowest of any EU country8. This demonstrates the potential of the EU to promote higher environmental standards in the UK which consequently have a positive impact on public health.
Tobacco Regulation
Responsibility for the sale, use and display of tobacco products is currently devolved to the Scottish Parliament. The Withdrawal Bill has listed tobacco regulation as a current EU competence that will return to Westminster. However, as public health is a devolved matter it is anticipated that this power will return to the Scottish Parliament.
Tobacco is one of the largest causes of health problems across Europe. In Scotland alone it is responsible for 1 in 5 deaths (10,000 a year) and costs NHS Scotland more than £300 million per year1. Tobacco regulation has strong, cross party support in the UK Parliament and its devolved counterparts.
EU wide regulation is currently controlled by the European Tobacco Products Directive (EUTPD 2014/40/EU). The directive takes into account recent scientific, market and international developments, and aims to improve the functioning of the internal market for tobacco and related products, whilst ensuring a high level of health protection for European citizens. This legislation made several changes to the sale of tobacco products in the EU: larger mandatory health warnings had to be included on every packet; cigarettes with characterising flavours were banned, along with promotional and misleading packets; and strict regulation and labelling for e-cigarettes was introduced. The UK went further than was required by prohibiting both point for sale displays and mandating standardised plain packaging of tobacco products.
A similar approach was taken with the European Tobacco Tax Directive (2011/64/EU) which sets out the minimum limits for tobacco excise duties. UK excise duties are significantly higher than the minimum and there is a commitment to increase them annually at 2% above inflation for the duration of this parliament2.
The European Tobacco Advertising Directive (2003/33/EC) prohibits cross border advertising, promotion and sponsorship by tobacco product companies. Again, the UK surpasses the requirements set by the EU by prohibiting all these practices domestically as well.
Post-Brexit these directives will probably be re-defined in legislation by both the UK Parliament and the Scottish Parliament after the initial implementation of the directives into UK law. Much of the current legislation on the regulation of tobacco is already domestic rather than European in origin. For example, the Scottish Parliament has passed legislation prohibiting smoking in public places, banning proxy purchasing for under 18s and raising the age of sale to 18.
The Association of Directors of Public Health (ADPH), in its evidence to the Health Committee at the House of Commons, highlighted that UK tobacco regulation has exceeded EU minimum requirements, and thus Brexit presents a possible opportunity for the UK to further regulate tobacco products. However, it has been suggested that there is a possibility that new trade agreements could weaken current safeguards3.
EURATOM
There is some concern regarding the UK's membership of the European Atomic Energy Community (EURATOM) post Brexit. EURATOM defines the safety standards relating to nuclear materials and aims to promote and support the development of nuclear energy in Europe. It monitors levels of radioactivity, as well as covering medical and occupational exposure to radiation and monitoring radiation in foodstuffs. Although EURATOM is established under its own treaty (The Euratom Treaty was signed in 1957 by the six founding States of the EEC (Belgium, France, Germany, Italy, Luxembourg and the Netherlands), it is governed by the EU institutions, including the CJEU. The Article 50 letter states that the UK intends to leave the European Atomic Energy Community1
One of the most important EURATOM directives is the Basic Safety Standards Directive 2013/59/EURATOM which is due to be implemented in the UK by February 2018. Other important directives include 2005/844/EURATOM concerning the accession of the European Atomic Energy Community to the Convention on Early Notification of a Nuclear Accident, and 89/618/EURATOM on informing the general public about health protection measures to be applied and steps to be taken in the event of a radiological emergency.
There are potential implications of the UK leaving EURATOM. First, it would cause disruption to the UK's nuclear industry. EURATOM regulates many aspects of the industry and it will take time to replicate these safeguards within the International Atomic Energy Agency. This is of particular concern to the UK as the UK has recently announced a multi billion-pound plan to build new nuclear power stations. Secondly, EURATOM regulates the import and export of radioactive and nuclear materials, importantly including medical isotopes used in the treatment of cancer. These materials cannot currently be produced in the UK, therefore there is the potential to create barriers between patients and the materials required for treating cancer which could result in delays to treatment. It is worth noting that the UK will have the ability to produce such isotopes at Hinckley Point Nuclear Power Station, from 20272. The UK Government says that it intends to legislate to pass on EURATOM's current roles to the UK regulator, the Office for Nuclear Regulation (ONR)3. However, there has been concern raised about the ONRs ageing workforce and it has been highlighted that it would be difficult for the ONR to take on any new responsibilities without recruiting a large number of staff4.
The Joint Report and Commission Communication outlined areas where there has been limited agreement in the first phase of Brexit negotiation. In relation to Euratom, the UK Government has introduced the Nuclear Safeguards Bill which would allow the UK Government to make regulations for, and implement international agreements in relation to, nuclear safeguarding. This is required once the UK leaves Euratom5.
Good Laboratory Practice
There are several EU directives relating to good laboratory practice (GLP) across member states. These are primarily aimed at promoting the quality and validity of data and results in the testing of chemicals in laboratories, and to prevent fraudulent practices.
Directive 2004/9/EC requires countries to establish authorities responsible for upholding GLP. It sets out the reporting and market requirement, including mutual acceptance of data requirements. It requires that several Organisation for Economic Co-operation and Development (OECD) guidelines are followed, including the guidance for Compliance Monitoring Procedures and the Conduct of Test Facility Inspections. This is supplemented by Directive 2004/10/EC which requires all member states to take the measures necessary to ensure that laboratories performing safety tests on chemical products are in line with OECD Principles of Good Laboratory Practice.
The potential impact of Brexit on this area of healthcare is likely to be minimal. As the EU directives were developed in accordance with OECD principles, and the UK remains a member of the OECD.
Public Sector Procurement
EU procurement rules harmonise standards for the purchase of goods, works and services by public authorities in order to create a level playing field for businesses from all member states. This is intended to contribute to increased quality and value for money. Tenders above a certain value must be advertised in the Official Journal of the EU and must be awarded impartially without discrimination in favour of local suppliers. For lower value contracts, national rules apply. EU law does not require Governments to open-up public sector services that provide a purely social function to the independent sector, nor does it prevent services that have been opened to competition from being returned to the public sector1. These rules are transposed into Scots Law by The Public Contract (Scotland) Regulations 2015 No. 446, and The Concession Contracts (Scotland) Regulations 2016 No. 65
NHS Scotland, having moved away from the internal market model, has been less impacted by EU procurement law than NHS England which has, to a greater degree, embraced private sector involvement in health. EU legislation may be seen as something helpful to Scotland in that it has been within this framework, including extra high thresholds for health contracts, that Scotland protected its NHS from private sector competition.
Procurement is not reserved under the Scotland Act 1998 but the Law Society of Scotland has voiced concern that the UK Government may wish to regulate public procurement at a UK level, potentially creating some uncertainty for NHS Scotland, particularly as the UK faces pressure in trade talks with other countries to open up all potential markets. They conclude:
It is vital that such powers, post-Brexit, are devolved to the relevant Governments to preserve the autonomy of healthcare provision in Scotland.2
A view that the fragmentation of procurement policy across the UK may not be best for transparency, competition and value for money has also been expressed3.
At a UK level, some potential opportunities from Brexit have been highlighted in respect of procurement. A briefing paper from the UK Trade Observatory at the University of Sussex observed:
this opens up the possibility of pursuing horizontal policy objectives, such as promoting SME [small and medium enterprises] or green public procurement.
It also makes reference to socially responsible public procurement and cites an EU study showing that the UK was already leading the EU in this area4.
In terms of transitioning out of the EU, the Nuffield Trust has expressed concern that:
a scenario where the UK leaves without any deal would cause extensive problems for the NHS. It would risk a chaotic disruption to supplies of medical products, and a rise in prices that would push hospitals deeper into deficit.
5
As to the future of health procurement, it is worth noting that the UK Government has positioned itself as a global champion of free trade6 and may therefore be unlikely to take steps that could be seen as protectionism in direct contradiction to this stated aim. The Nuffield Trust has said:
trade deals along the lines the Government plans, either with the EU or with other countries, may make it difficult to change procurement or competition law even after we leave the EU.5
Perhaps in anticipation of such a scenario, the BMA 8is calling for wholesale reform to end open competition in health. Their latest briefing recommends that:
The UK Government should end the application of EU competition and procurement law to the commissioning of NHS services in England.
Access to NHS markets should not be used as a bargaining chip in relation to any future trade deals with the EU.
The NHS should be exempted from any future international trade deals, such as those with the USA.
Post-Brexit, all regulations and rules requiring competitive tendering within the NHS or HSCNI services should be removed, allowing clinicians and commissioners to focus on care and not competition8.
Blood, Organs, Tissues and Cells
The EU has legislated on blood, organs, tissues and cells on several occasions over the last 15 years. The main directives EU are aimed at ensuring high levels of public health protection, through setting quality and safety standards, while also facilitating increased exchanges of these substances between member states. Spending on blood, tissues and cells is estimated at €6 billion per year across the EU1.
EU standards apply to the various steps, from donation to transfusion/transplant, as well as to those persons/organisations responsible for such activities. The directive on organs outlines the need for: the appointment of ‘Competent Authorities’ in all member states; for the authorisation of procurement and transplantation centres and activities; for traceability systems; as well as for the reporting of serious adverse events and reactions; and requirements for the safe transportation of organs.
Regulation of clinical application and ethical decisions, for example on donor consent systems and access to treatment, remains at a member state level and each member state is expected to implement licensing, inspection and reporting schemes.
The Medicines and Healthcare products Regulatory Agency (MHRA) regulates blood components for safety and quality. The UK regulatory body, the Human Tissue Authority (HTA), acts as the competent authority for Scotland. The HTA regulates organisations that remove, store and use human tissue for research, medical treatment, post-mortem examination, education and training and display in public. It also gives approval for organ and bone marrow donations from living people. NHS Blood & Transplant (NHSBT) manages the UK Organ Donor Register and the National Transplant Register and is responsible for transplant services across the UK.
The Scottish National Blood Transfusion Service (SNBTS) is licensed by the MHRA in respect of all blood activities and cellular therapy medicinal products and the HTA in respect of tissue and cell activities. It recruits and cares for donors, collects and maintains supplies of blood, tissues and cells and provides diagnostic, matching and treatment services.
In practical terms, following Brexit, the UK Government and/or devolved administrations will have to take on additional responsibility for policy development in terms of quality and safety of blood, organs, tissues and cells.
A key consideration will be whether or not to maintain parity of standards with the EU. This is an important issue if the UK wishes to be able to maintain supplies and match donors in the EU27. Figures from NHSBT’s Annual Transplant Activity Report for 2016-17 show that, in the last three years, 50 solid organ transplants have taken place from deceased UK donors to EU recipients and a further 74 transplants have taken place from deceased EU donors to UK recipients. To put these figures in context, the total number of deceased solid organ donors in the UK for 2016-17 was just over 1,400
A key concern will be to maintain supply of safe, quality assured blood, organs, tissues and cells to Scottish patients. Writing in the Lancet, Fahy, McKee et al. express concern:
More complex issues, such as securing human blood, organs, or tissue supplies are also subject to specific provisions in EU law and are likely to face difficulties and short-term disruptions.2
The Anthony Nolan Trust, the UK’s stem cell registry stated:
“When regulations are aligned they enable us to work quickly and efficiently to provide donors, and as such if UK and EU regulations become increasingly divergent this may have a serious impact on our day-to-day work as a stem cell registry.”
Seeking to alleviate concerns about health regulation post-Brexit, the UK Secretary of State for Health, Jeremy Hunt, and the Secretary for Business, Greg Clark, wrote a joint letter to the Financial Times in July stating:
Whatever the outcome of Brexit negotiations, we are clear that should we not achieve our desired relationship with the EU, we will set up a regulatory system that protects the best interests of patients and supports the UK life science industry to go from strength to strength 3
Food Compositional Standards and Labelling
A number of EU Regulations are in place to inform and protect consumers and prevent misleading practices including: the provision of food information to customers; the legal framework in respect of nutrition and health claims on food labelling and advertising; addition of ingredients; and food for specific groupsi.
Food compositional standards are not reserved under the Scotland Act 1998, unlike other aspects of consumer protection. Although, regulations have direct effect and do not require implementing legislation, the Food Information (Scotland) Regulations 2014 (as amended) provide for enforcement. The Food (Scotland) Act 2015 established Food Standards Scotland, the regulatory body tasked with developing and advising on food policy as well as monitoring enforcement of the regulations by local authorities.
Key issues for consideration post-Brexit may include:
The relative costs and benefits of seeking to maintain EU standards, in particular, the potential impact on public health should standards be reduced.
Whether food compositional and labelling standards are best set at devolved or UK level and which organisation will take on the role currently played by the EU in terms of policy and legislation.
While there has been significant media commentary on the potential impact of leaving the EU, for food production and trade there has been less of a focus on the implications for public health.
The University of Sussex Science Policy Research Unit considered the health implications of a departure from EU standards1 they noted:
In the EU, UK consumers and public health have benefited from EU-wide safety standards, without which there will be a risk of the UK having less safe and nutritious products.
The report points out that, while EU legislation requires the presence of GM ingredients to be labelled, if over 1% of the relevant material, the USA has no such labelling requirement. Similar concerns are described in relation to beef from animals that received hormone treatment or chicken that has been dipped in disinfectant, a practice not lawful in the EU. EU rules on the use of chemicals in food production currently limit imports of meat, poultry and dairy, produced in countries with less stringent rules.
A House of Lords Committee report in July 20172 highlighted the dangers of deregulation post-Brexit in terms of the implications for farming and animal welfare standards. Concerns have been further aired in the media about whether UK international trade deals after Brexit could result in pressure on the UK to accept food imports produced to lower standards, potentially impacting on health and putting pressure on Scotland’s producers.
Responding to concerns, a No. 10 Downing Street spokesman said:
Our position when it comes to food, is that maintaining safety and public confidence in the food we eat is of the highest priority.3
Countering suggestions that deregulation was likely post-Brexit the Chief Executive of Scotland Food and Drink said:
There are some who think coming out of the EU will be the catalyst for the unwinding of a huge amount of regulation. I think that’s a pipedream…I don’t think that’s a path we want to go down. The 96 per cent of our Scotch beef exports that go to the European Union, if we want that to continue, we absolutely have to abide to EU minimum standards…4
The Food and Drink Federation's manifesto published in June does address regulation. It includes a call for:
A stable regulatory framework through the Great Repeal Bill and other legislation. Maintaining consumer confidence in the safety and authenticity of UK food and drink is paramount. We must protect the UK’s reputation for high quality products, while where possible, boosting the competitiveness of our sector.5
Similarly, the Scottish Organic Producers Association (SOPA) says that 90% of its members want no change in the regulation of organic foods6.
As Scotland has its own legislation, regulatory and enforcement mechanisms around food, implementing EU standards, these are not expected to change post-Brexit. With a large proportion of Scotland’s food exports going to the rest of the UK it is expected that there will be a focus on facilitating trade within the UK.
Data Protection
Rules to protect personal data are essential in the digital age. Given its responsibilities for managing large volumes of personal and sensitive data, the implications for health and social care are significant. The EU began a review and reform of its data protection legislation in 2012 to strengthen citizens’ fundamental rights while simplifying the rules. New legislation was agreed in 2016. Regulation (EU) 2016/679 (General Data Protection Regulation) (GDPR) on the protection of natural persons with regard to the processing of personal data and on the free movement of such data and will apply from 25 May 2018.
An update to the ePrivacy Directive 2002 was proposed on 10 January 2017; once adopted, the ePrivacy Regulation will update the “rules of the road” for privacy and electronic communications. It will modernise existing principles, clarify the technological requirements and provide for effective enforcementi.
The GDPR will automatically come in to effect in the UK on 25 May 2018. However, as the UK Government wishes to maintain the ability to share data with EU states, post-Brexit, it has introduced its own Data Protection Bill. The extent to which this aligns with the GDPR will be crucial if the UK is to continue to share data with the EU for example, with a view to researching and combating disease.
Post-Brexit, the UK will have to abide by the EU rules for personal data transfer to third countries (countries outside the EU and EEA) if it wishes to participate in the sharing of such data. This data can only be transferred if the EC deems that there is an adequate level of protection. Options for satisfying this condition include adequacy decisions made by the EC, binding corporate rules and standard contractual clauses. However, as the GDPR will automatically apply on 25 May 2018, the UK should remain compliant post-Brexit unless this is altered by subsequent legislationii.
Potential issues arising from Brexit include regulation of life sciences research and pharmaceutical trials which rely on the UK being compliant with EU Data Protection legislation, particularly with reference to research in genomic medicine and rare disease research. The European Reference Network (ERN) consists of clinicians and other healthcare professionals who collaborate on specific complex and rare conditions, by sharing knowledge and expertise that would not be available locally or even nationally. By creating a large pool of patient data, ERNs can facilitate clinical trials and develop new drugs, creating potential for better outcomes for the patients. There is a potential for this work to be disrupted by Brexit, if the UK is not able to satisfy third country obligations for data sharing12.
Cross border employees could also be impacted on as personal data can only be transferred if a third country's protection is deemed adequate by the EC, so all potential employers and recruiters of EU nationals will have to be aware of the options available to them (e.g. binding corporate rules, standard contractual clauses) and be ready to meet them if required. With regard to reciprocal healthcare, the implications for the sharing of patient records will have to be explored.
Finally, Article 9 of the GDPR covers the circumstance under which the processing of personal data, including health data, genetic data, and biometric data is permitted. It states that member states may maintain or introduce further conditions.
Parliamentary Work
A number of UK Parliamentary committees have carried out inquiries into the implications of Brexit on health and social care. These include:
House of Commons Select Committee on Health (2017) Brexit and health and social care – People and Process
House of Commons Scottish Affair Committee (2017) Scotland's Place in Europe Inquiry Written Evidence
House of Lords EU Home Affairs Sub-Committee (2017) - Brexit Reciprocal Healthcare Oral Evidence
House of Commons Science and Technology Committee (2016) Leaving the EU: Implications and Opportunities for science and research.
The Scottish Parliament's Health and Sport Committee has been carrying out work to investigate the effect that Brexit will have on health and social care. The committee has agreed within its strategic plan to “test all activity we scrutinise against… the implications of the UK's EU exit”.
The Committee has agreed to undertake an inquiry to consider what the NHS and social care in Scotland could look like post-Brexit with a focus on how potential risks could be mitigated and potential opportunities could be realised. A call for written evidence had been issued which closed on the 25 January 2018. In response to this, the Committee received a letter from the Cabinet Secretary for Health and Sport which outlines concerns regarding some of the potential implications of Brexit for the health and social care sector in Scotland. It also reiterated her commitment to provide regular updates to the Committee on the impact of Brexit on health and social care.
Annexe A: Scottish NHS Boards and Local Authority Workforce Responses
SPICe contacted all 14 territorial health boards and the 32 local authorities in Scotland to enquire about the number of non- UK EU nationals in their workforce.
NHS Boards were asked the following questions:
For your health board would you be able to provide:
Information on the total number of staff and number who are non-UK EU nationals, by staff group.
If this is not available please could you:
provide any estimates you have on the number of non-UK EU nationals employed
or reply stating that this information is not available.
| NHS Board | Response | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NHS Ayrshire & Arran | We are able to provide an estimate of the data requested, taken from the personal information forms completed by staff when they start with the organisation. Completion of this section is not compulsory, so it may not give a complete picture of the overall staff profile in terms of non-UK EU nationality. The numbers, as of 7th November, are as follows:
| ||||||||||||||||||||||||||||||||||||
| NHS Borders | On the basis of limited data on the nationality of employees from their records, the following table provides estimated figures for non-UK EU nationals employed by NHS Borders.
| ||||||||||||||||||||||||||||||||||||
| NHS Dumfries & Galloway | No response | ||||||||||||||||||||||||||||||||||||
| NHS Fife | We are unable to provide the information requested.Discussions are currently underway with colleagues within the Scottish Government workforce directorate with regard to how Board’s can approach any potential options for capturing this data on a consistent basis across NHS Scotland including consideration of the Health and Social Care Partnership context. | ||||||||||||||||||||||||||||||||||||
| NHS Forth Valley | This information is not available and we do not have an estimate. | ||||||||||||||||||||||||||||||||||||
| NHS Grampian | I confirm that NHS Grampian does not record this information. | ||||||||||||||||||||||||||||||||||||
| NHS Greater Glasgow & Clyde | NHSGGC does not currently collect country of origin for any member of staff recruited from within the EU – whether they are from the UK or out with – therefore I am unable to provide any reasonable estimate of the numbers.I have extracted data held on the NHSGGC Human Resources system however only 5% of our workforce have their nationality recorded. Robust information is held where staff require a VISA to work within the UK however as this is not required for people moving within the EU it was not deemed a necessary piece of information to capture at point of recruitment. | ||||||||||||||||||||||||||||||||||||
| NHS Highland | We are unable to provide the information that you have requested as it is not available. | ||||||||||||||||||||||||||||||||||||
| NHS Lanarkshire | Does not hold information on employee nationalities but did provide data on Ethnic Group instead. | ||||||||||||||||||||||||||||||||||||
| NHS Lothian | No response | ||||||||||||||||||||||||||||||||||||
| NHS Orkney | NHS Orkney does not presently record what country members of staff are from. | ||||||||||||||||||||||||||||||||||||
| NHS Shetland | NHS Shetland is unable to provide a comprehensive breakdown of non-UK EU nationals working within the board. However, the board is aware of 2 non-UK EU nationals employed by the board at present:1 x Support Services Staff1 x Allied Health Professions | ||||||||||||||||||||||||||||||||||||
| NHS Tayside | NHS Tayside does not record non-UK EU nationals, only non-UK non-EU nationals. | ||||||||||||||||||||||||||||||||||||
| NHS Western Isles | The total number of staff employed by NHS Western Isles was (at 31st October 2017) 1007 (headcount)In NHS Western Isles we do not currently collect and record information about nationality of staff in a way that can identify the numbers of non-UK EU nationals. | ||||||||||||||||||||||||||||||||||||
Local authorities were asked:
For your local authority would you be able to provide:
Information on the total number of social care staff and number who are non-UK EU nationals, by staff group.
If this is not available please could you:
provide any estimates you have on the number of non-UK EU nationals employed
or reply stating that this information is not available.
| Aberdeen City Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Aberdeenshire Council | Total number of social care staff: 2,440 (this figure includes all social care staff within H&SC Partnership)Number of non-UK EU nationals: 56 (by job type and service area)
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Angus Council | Angus Council currently has 745 social care staff. In terms of the number who are non-UK EU nationals, this information is not available. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Argyll and Bute Council | We presently have 113 Social Care staff of which 18 are Scottish, 2 are Other and 93 is blank. Please note that it is not compulsory for staff to confirm their nationality. | ||||||||||||||||||||||||||||||||||||||||||||||||
| City of Edinburgh Council | The total number of staff in the roles below is 2,288 and we estimate that a total of 157 non UK EU nationals are currently working in our Health & Social Care Department in the following Social Care roles. Post Title Number of PeopleCare and Support Worker 22Care Coordinator 3Community Therapy Assistant 6Home Care Organiser 12Occupational Therapist 5Other Social Care Role 2Senior Care and Support Worker 3Social Care Assistant 34Social Care Worker 62Social Worker 3Support Worker 5 Grand Total 157To promote transparency and accountability, please note it is the Council’s policy to publish all request details and responses made under the freedom of information legislation. This information will be made available through the Council’s website and will not include your personal details. The disclosure log is available at the following link: http://www.edinburgh.gov.uk/homepage/175/foi_disclosure_log | ||||||||||||||||||||||||||||||||||||||||||||||||
| Clackmannanshire Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Comhairle nan Eilean Sair | We are unable to provide this information on non-UK EU Nationals in the format you require as we do not hold this information, however we are able to provide an overview of employee Nationality/Citizenship gathered through Equality Monitoring which is detailed in the table below. You will note that a large percentage of our employees have not disclosed their Nationality/Citizenship, and of those who have, our numbers for non-UK EU Nationals is small.The total Headcount for Social and community services is 503 Employees.Nationality/Citizenship Employees %Scottish 973 46.9%English 16 0.8%Irish * *Welsh * *UK 9 0.4%GB 6 0.3%British 318 15.3%Italian * *American * *Indian * *Australian * *Canadian * *Romanian * *German 5 0.2%Nepali * *Latvian * *Other * *PNTA * *Not disclosed 725 35.0%Total 2073 100.0%Please note: Table is based on all contracted staff and (*) = less than 5 Employees | ||||||||||||||||||||||||||||||||||||||||||||||||
| Dumfries and Galloway Council | Of the 601 social care staff the breakdown is as follows:UK Nationals 559Other Ethnic Groups or non-disclosed/not known 39Non UK EU Nationals 3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Dundee City Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| East Ayrshire Council | East Ayrshire HSCP currently have 678 Personal Carers/Senior Carers employed, 6 of which are non UK EU Nationals. | ||||||||||||||||||||||||||||||||||||||||||||||||
| East Dunbartonshire Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| East Lothian Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| East Renfrewshire Council | Unfortunately we do not have that specific information as we record the ethnicity of our employee rather than nationality.However we employee approximately 630 employees in our Health & Social Care Partnership. Over 450 of these employees have declared themselves to be White Scottish. Of the remainder I do not think there is a significant number who are non- UK EU Nationals. Our estimate is single figures – perhaps as low as 5 employees. At this stage East Renfrewshire Council does not envisage Brexit having a major impact on our Social Care sector. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Falkirk Council | I can advise that we do not currently hold information on the number of our social care staff who are non-UK EU nationals. As there was no work restrictions for this group we did not previously capture this information. Whilst we have some employee ethnicity data, it does not currently capture nationality. We are however in the process of collating data to estimate the number of non-UK EU nationals within our overall workforce. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Fife Council | Fife Council does not hold the information you requested in terms of Section 17 of the Act – Information not held. We do not hold the nationality of our employees on any of our systems, we only hold ethnicity.For assistance, we can advise that in November 2016 we received a similar enquiry for the number of EU nationals we have working in the Council’s Social Work service, including children and within NHS Fife. As far as we could establish from the Disclosure Scotland information which covers Education, Social Care and other registered employments within the council, those identifying themselves as EU was around 2%. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Glasgow City Council | I write in response to the above enquiry on behalf of Glasgow City Council. I’m afraid that we retain no data on the nationality of employees except for that required by law and pertaining to non-EU nationals. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Highland Council | The Highland Council does not currently hold this information, nor do we have an accurate estimate. Please note that we are currently considering the options available to gather this type of information from staff and would be interested in how other public sector bodies have approached the task. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Inverclyde Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Midlothian Council | I regret to advise that we are unable to assist in providing you with the number of social care staff employed by local authority that are non-UK EU nationals.Staff are not required to provide equalities data and therefore we do not have the information you have requested. From the data we do have I can confirm that the Council employs 489 Adult and Social Care staff.This is broken down as follows:British 119Scottish 159English 3Northern Irish 1Unknown 207Total 489 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Moray Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| North Ayrshire Council | Provided data showing that of the 557 staff where nationality was known, 4 were EU nationals. Nationality was unknown for the majority of staff although (n=814 of the 1371 total headcount) | ||||||||||||||||||||||||||||||||||||||||||||||||
| North Lanarkshire Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Orkney Islands Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Perth & Kinross Council | Provided figures of 6 non-UK EU nationals within their 738 workforce (0.8%), although 70 employees did not declare their nationality so it may be higher than indicated. Also provided results of s survey of the independent care sector and found the following:Of the 45 care homes surveyed, 17 responded with the following: - Estimated total number of staff - 879 - Estimated total number of staff from EEA countries - 88 - Estimated total number of staff other/not known - 39Of the 13 independent care at home providers, 6 responded to the survey with the following: - Estimated total number of staff - 306 - Estimated total number of staff from EEA countries - 24 - Estimated total number of staff from other/not known - 0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Renfrewshire Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Scottish Borders Council | We have 1012 employees recorded as working within Social Work and SB Cares. We have 6 recorded as non UK EU nationals working as Support Workers within SB Cares. Please note diversity information is requested from each employee, it is their decision whether or not to complete. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Shetland Islands Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| South Ayrshire Council | The estimated number of non-UK EU nationals employed in social care by this Council is 3. | ||||||||||||||||||||||||||||||||||||||||||||||||
| South Lanarkshire Council | I can confirm that our records show that we have fewer than 20 non UK EU nationals working within social care. I can confirm that South Lanarkshire Council is planning for employees to update their records with this information in due course. Please let me know if you require any further information on this matter. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Stirling Council | On reviewing our diversity information I estimate there are 94 employees affected by Brexit and in relation to the Health and Social Care workforce the number is 13 employees. The following is a list of areas of work;MENTAL HEALTH TEAM ADULT SOCIAL CARE SUPPORT ALLAN LODGE - CARE HOME OUTREACH SERVICES HOME CARERS ADULT SUPPORT & PROTECTION MECS TELECARE ASSESSMENT & CARE MANAGEMENT MENTAL HEALTH TEAM LOCALITY 1 TEAM SENSORY IMPAIRMENT CRIMINAL JUSTICE SERVICE HOME CARERS | ||||||||||||||||||||||||||||||||||||||||||||||||
| West Dunbartonshire Council | No response. | ||||||||||||||||||||||||||||||||||||||||||||||||
| West Lothian Council | No response. |
[1] Workforce figures for NHS Scotland and each health board