Report on petition PE1463: Effective thyroid and adrenal testing, diagnosis and treatment (revised on 20 July 2018 to update glossary)
Glossary of terms
To assist readers of this report, we have included a glossary of terms in two sections. The first section provides information about the various organisations referred to in the report and the second section provides information about clinical or scientific terms.
Organisations
| British Thyroid Association | A non-profit learned society of professional clinical specialist doctors and scientists in the UK who manage patients with thyroid disease and/or are researching into the thyroid and its diseases in humans. |
| British Thyroid Foundation | A UK patient support charity for patients with thyroid disorders. |
| National Institute for Health and Care Excellence (NICE) | A non-departmental public body that provides national guidance and advice to improve health and social care in England.iii |
| Royal College of General Practitioners | The professional membership body for family doctors in the UK.iv |
| Royal College of Physicians | The professional membership body of physicians in the UK.v |
| Scottish Clinical Biochemistry Managed Diagnostic Network | A national body that comprises staff working in clinical biochemistry laboratories in all 14 NHS Scotland Health Boards.vi |
| Scottish Intercollegiate Guidelines Network (SIGN) | A national body that develops evidence based clinical practice guidelines for the NHS in Scotland.vii |
| Thyroid UK | A registered charity, primarily concerned with the provision of quality information for people with both diagnosed and undiagnosed thyroid disorders, working through dialogue with NHS departments, networking with other voluntary organisations and providing services direct to patients.viii |
Clinical and scientific terms
| Thyroid | A small, butterfly-shaped gland located in the neck and which produces three main hormones: thyroxine (T4), triiodothyronine (T3) and calcitonin. The first two hormones affect the body’s metabolism by increasing the metabolic rate. T3 can be made in the thyroid from the breakdown of T4 and also by the same mechanism in other tissues in the body. Calcitonin is responsible for controlling the levels of calcium and phosphorus in the blood.ix |
| Thyroid Stimulating Hormone (TSH) | A hormone made by the pituitary gland (an organ located below the brain and behind the sinus cavities). When concentrations of thyroid hormones decrease in the blood, the hypothalamus (an organ in the brain) releases Thyrotropin Releasing Hormone (TRH). This stimulates the release of TSH by the pituitary gland. The TSH in turn stimulates the production and release of T4 and T3 by the thyroid gland.x |
| Hyperthyroidism | Over-activity of the thyroid gland.x |
| Hypothyroidism | Under-activity of the thyroid gland.x |
| Thyroid Stimulating Hormone (TSH) Test | A test that measures the amount of thyroid stimulating hormone in the blood.x |
| Free T3 (free triiodothyronine) test | The test measures the amount of free triiodothyronine (FT3) in the blood. The test determines whether the thyroid is performing properly and is used mainly to help diagnose hyperthyroidism since FT3 can become abnormally higher earlier than FT4 and returns to normal later than FT4. FT3 is not usually helpful in the diagnosis of hypothyroidism. |
| Free T4 (free Thyroxine) test | A test to diagnose hypothyroidism or hyperthyroidism in adults and to monitor response to treatment. An abnormal TSH is usually the indicator for an FT4 test to be carried out. A high FT4 result may indicate hyperthyroidism and a low FT4 result may indicate hypothyroidism. FT4 is the free hormone that is thought to be responsible for all the effects of the thyroid hormone within the body. Measurements of TSH and FT4 would be used to check the progress of patients undergoing treatment for hypothyroidism. |
| Total T3 test (TT3) | A test that measures the total levels of triiodothyronine levels (both bound and free) in the blood. |
| Total T4 test (TT4) | A test that measures the total levels of thyroxine levels (both bound and free) in the blood. |
| Reverse T3 (RT3) Test | A test that measures levels of Reverse T3, a biologically inactive form of T3 produced in the liver. Levels of RT3 may be elevated in both hypothyroidism and hyperthyroidism. The clinical effectiveness of the RT3 test is contested.x |
| Adrenal Stress Index Test | A salivary test that measures levels of cortisol and dehydroepiandrosterone. This is used to evaluate adrenal function and fluctuations in the circadian rhythm.xv |
| Levothyroxine (‘L-T4’) | The standard synthetic hormone treatment for hypothyroidism in the UK.xvi |
| Liothyronine (‘L-T3’) | A thyroid hormone produced in the UK. Until summer 2017, the pharmaceutical company Amdipharm Mercury Company Ltdxvii held the only licence in the UK to produce this medication. |
| Natural desiccated thyroid | A medicine not licensed for use in the UK, which is extracted from porcine thyroid glands.xviii |
Executive Summary
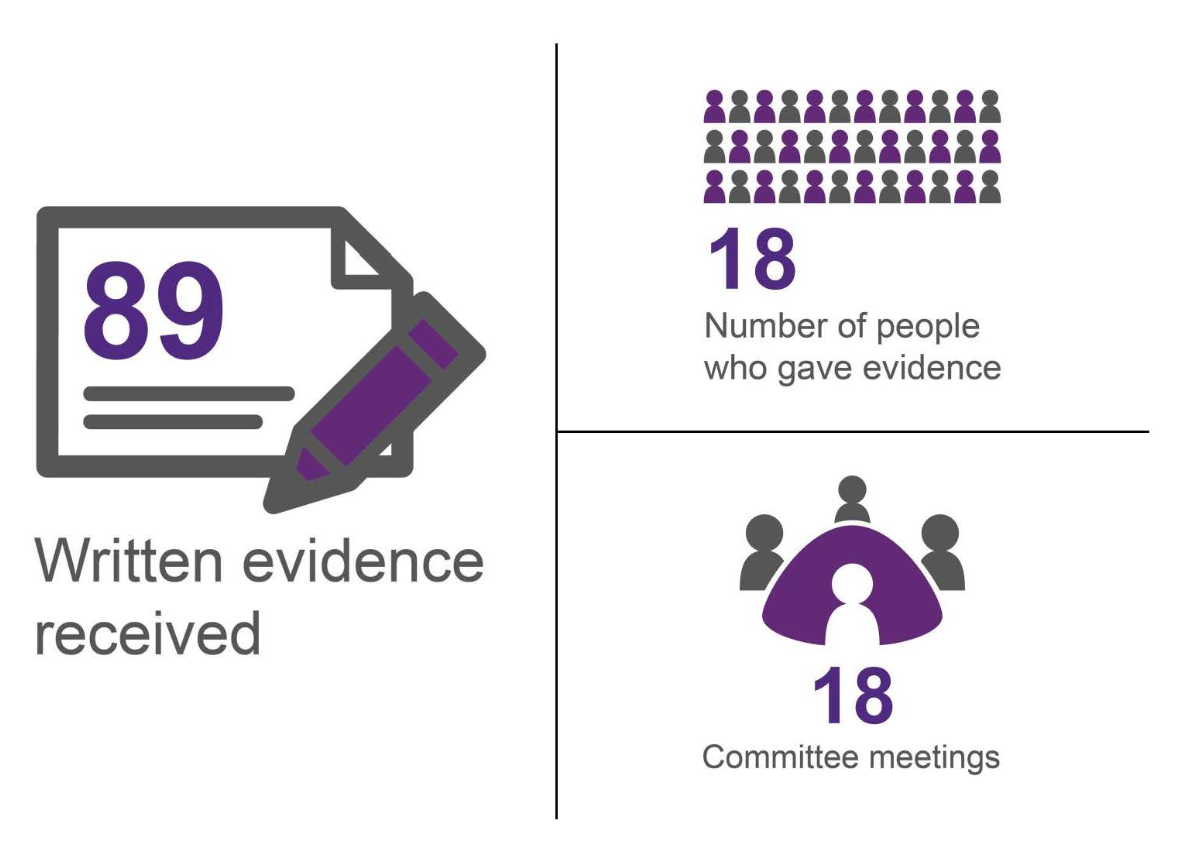
Consideration of PE1463 has spanned five years. In that time a significant range and volume of evidence has been gathered, both by way of written submissions and oral evidence. This work is illustrated in Figure 1.
In seeking to understand and scrutinise the issues raised by the petition, the current Committee and its predecessors have taken evidence from the petitioner, the Scottish Government, public bodies, clinical representatives and thyroid patient representatives. Written evidence has been received from a similar range of stakeholders. Elaine Smith MSP has maintained an interest in the petition throughout its consideration and has contributed to many of the meetings at which the Committee has considered this petition.
The Committee is grateful to all those who have contributed to consideration of this petition since 2012; their input has greatly assisted the Committee in reaching its conclusions and recommendations.
Summary of conclusions and recommendations
Guidance Framework
It is clear to the Committee that clinicians have an important, independent role in diagnosing and treating patients. In carrying out this duty, clinicians are guided by evidence-based guidelines. These guidelines are developed by independent experts (health authorities and clinical bodies) and are informed by the available peer-reviewed research on patient treatment.
The Committee welcomes the fact that the profile-raising brought about by the petition appears to have been influential in key sources of clinical guidance being produced or updated by professional bodies and national health authorities.
The British Thyroid Association's acknowledgement that some patients continue to experience ill-heath on standard treatment is a positive step forward in bringing this issue to the attention of clinicians and mainstream endocrinology. The Committee welcomes the British Thyroid Association's steps in consulting with a patient support charity in the process of developing its position statement.
The Committee considers it essential that professional bodies continue to work closely with patient support groups and individuals, such as the lead petitioner, so that greater awareness of guidance and patient experience is promoted.
The Committee recommends that the Scottish Government ensures bodies within Healthcare Improvement Scotland also contribute to this work and that, in responding to this report, it provides an outline of how that contribution can be made.
Diagnosis and testing
The Committee recognises the importance of accurate and accessible public information for patients to inform their treatment decisions and options. In this regard, the Committee is pleased that its work has resulted in updated public information regarding different testing options being made available on Lab Tests Online.
The Committee recommends that the Scottish Government brings the evidence received on the variation in thyroid testing in Scotland to the attention of the Scottish Clinical Biochemistry Managed Diagnostic Network. In doing so, the Committee suggests that consideration should be given to moving towards the development of a single, national protocol for testing, along with an accompanying process for issuing consistent advice to primary care practitioners for use when considering and interpreting diagnostic tests for suspected hypothyroidism.
Treatment
The Committee recognises the Government's role in relation to treatment is to provide policies, frameworks and resources to National Health Service boards to allow them to deliver services that meet the needs of their local populations. In doing so, it is vital that consideration is given to clinical evidence, knowledge and guidance such as that offered by bodies like the British Thyroid Association or NICE.
The Committee considers that the clinical information referred to in this report now makes clear the treatment options that can be considered by doctors and patients, as well as clarifying the position on the prescription of unlicensed treatment options, such as natural desiccated thyroid.
The Committee recognises the concerns that the petitioner has raised about the issue of supply of T3. The Committee notes that the licensing of medication is reserved but that the Government's action has included discussing previous supply issues with the UK Department for Health to monitor the supply of T3 in the UK. It understands that the issue of temporarily sourcing unlicensed supplies of the medication have since been addressed.
In relation to the production and costs of T3, the Committee understands that the market for this treatment in the UK is relatively small. For the majority of the period in which this petition has been under consideration, there has been only one licensed supplier of T3. However, the Committee understands that two further licences were granted in summer 2017. The Committee asks the Scottish Government, in its response to this report, to provide information about the impact that the granting of further licences may have on the costs of T3. The Committee asks this particularly with regard to the Competition and Markets Authority's recent announcement of a provisional finding that Concordia has overcharged the NHS by millions of pounds for T3.
Finally, on the issue of natural dessicated thyroid, the Committee notes that this is not currently licensed and understands there are different views about its efficacy as a treatment for hypothyroidism. The Committee is not aware of any current applications for natural dessicated thyroid to be licensed but draws the attention of the MHRA to the anecdotal evidence received on this petition and the number of people who report that use of natural dessicated thyroid leads to an improvement in their symptoms.
Research
In the Committee's view, better means of capturing patient experience need to be developed so that clinicians can deliver the Scottish Government's aim to promote ‘realistic medicine’ that ensures the most appropriate treatment is given to patients. In this regard, the Committee recommends that the Scottish Government should develop guidance for listening exercises to ensure that they are designed in an impactful way and obtain best value for money.
This includes ensuring that the views of the relevant target group can be extrapolated from the results, for example, ensuring that Scottish patients’ views can be clearly identified from the results where participants who are not Scottish-domiciled also take part. Listening exercises should also be designed to capture ‘anecdotal’ evidence in a way that can meaningfully inform the development of future clinical studies, clinical guidance or public policy (whichever is relevant to its aims). It is also recommended that the Scottish Government ensures that the participants receive feedback on the outcome of the listening exercise, including any specific action points that will be taken forward.
Overall conclusion
Thyroid conditions can have a significant impact on the lives of those who are diagnosed with having them. While the majority of patients with hypothyroidism will be diagnosed and treated successfully using the standard treatment and testing regimes, there is a proportion of patients for whom this is not the case.
This petition has raised the profile of that cohort of patients who either do not respond to the standard treatment, or who do not respond to the extent that they feel sufficiently well. It is important that these patients are believed when they report ongoing symptoms to clinicians.
However, the Committee recognises that there are differing views as to the evidential basis of both the current system of diagnosis, testing and treatment and the changes that are called for in the petition. The Committee recognises that discussions about these differing views will, and should, continue. This report sets out the Committee's conclusions and, where appropriate, makes recommendations for consideration by the Scottish Government and other decision makers. The Committee looks forward to receiving responses from these bodies.
Introduction
PE1463 by Lorraine Cleaver, Marian Dyer and Sandra Whyte was lodged on 19 December 2012. The petition calls for the Scottish Parliament to urge the Scottish Government to take action to ensure GPs and endocrinologists are able to accurately diagnose thyroid and adrenal disorders and provide the most appropriate treatment.
While the petition is drafted in broad terms to cover all adrenal and thyroid disorders, the petitioners explained in evidence to the Committee that their petition is focused on a particular subset of hypothyroid patients:
By definition, primary hypothyroidism is a problem with the thyroid gland, but the condition we are talking about is not with the thyroid gland. It is about the hormone that comes from the thyroid gland that becomes inactive…the condition we are talking about today [is] a conversion failure of the inactive T4 thyroid hormone to cross over into the active T3 hormone.i
The Public Petitions Committee's scrutiny of the issues has therefore focused on a subset of thyroid and adrenal diseases – hypothyroidism and those patients in particular who continue to experience symptoms while undergoing the recommended standard treatment.
The National Institute for Health and Care Excellence's (NICE) Clinical Knowledge Summary on hypothyroidismii provides an overview of the condition:
Hypothyroidism is the result of reduced production of thyroid hormones (thyroxine [T4] and tri-iodothyronine [T3]). Thyroid hormones are released from the thyroid gland when it is stimulated by thyroid-stimulating hormone (TSH) from the pituitary gland.
Primary hypothyroidism (95% of cases) occurs when the thyroid gland is unable to produce thyroid hormones because of iodine deficiency or an abnormality within the gland itself. It presents as:
Overt hypothyroidism: TSH levels are above the normal reference range (usually above 10 mU/L) and free T4 is below the normal reference range.
Subclinical hypothyroidism: TSH levels are above the normal reference range and T3 and T4 are within the reference range.
Secondary or central hypothyroidism is the result of insufficient production of TSH due to a pituitary or hypothalamic disorder.
Postpartum thyroiditis is the development of thyrotoxicosis and/or hypothyroidism, within a year of giving birth in women who had normal thyroid function prior to pregnancy.
In the UK, hypothyroidism is most commonly caused by autoimmune disease or thyroid damage as a result of surgery or radioiodine therapy.
The most common cause of hypothyroidism is the autoimmune condition, Hashimoto’s thyroiditis. Both hyper- and hypothyroidism can also be caused by thyroiditis (thyroid inflammation), thyroid cancer, and excessive or deficient production of TSH.
According to NHS Choices, both men and women can have an underactive thyroid.iii In the UK, it affects 15 in every 1,000 women (1.5%) and 1 in 1,000 (0.1%) men. Children can also develop an underactive thyroid. According to NICE, the prevalence of spontaneous hypothyroidism in areas where there is adequate iodine in the diet (such as in Scotland) is 1–2%.iv
The petition background information highlights the petitioners’ four main concerns:
The inclusion of tests for Free T3 (FT3) and Reverse T3 (RT3) thyroid hormones, as these are the strongest indicators of cellular thyroid levels;
Medical professionals to acknowledge that adrenal insufficiency exists and to incorporate The Adrenal Stress Index Test within NHS thyroid testing procedures;
Medical professionals to acknowledge and take account of variations in individual biochemistry and tailor treatment accordingly. Treatment may consist of: T4 only; T4/T3; T3 only or natural desiccated thyroid – or whatever combination to suit the individual patient. They must also provide appropriate support for adrenal insufficiency; and
NHS procedures to include testing of autoimmune status, minerals, enzyme, and vitamins. The ‘active B12’ (methylcobalamin) is more effective than the current injection of hydroxocobalamin. Most Scots are vitamin D deficient, and must have high level replacement.
Guidance Framework
The petition calls for action to be taken to ensure that GPs and endocrinologists are able to accurately diagnose thyroid and adrenal disorders and to provide the most appropriate treatment. In evidence to the Committee on 5 February 2013, the petitioners clarified that their specific concern is for a particular subset of hypothyroidism patients; those who do not respond well to the standard treatment because they are not able to metabolise it.i
The lead petitioner, Lorraine Cleaver, explained during the evidence session why the development of clinical guidance is a key aim of the petition:
There are no guidelines in Scotland for the treatment or diagnosis of hypothyroidism. Everybody refers to the Royal College of Physicians policy document, but that was never requested by the health department – it is just the RCP's own policy document…However, those guidelines do not provide for people like us who do not convert T4.i
UK professional bodies' guidance
A starting point for the Committee was therefore to investigate the existing sources of information that are available to clinicians. The Royal College of Physicians (RCP) provides guidance to members on a range of conditions. Its submission of 11 September 2013 did not comment on the action called for by the petition but referred the Committee to its position statement entitled “the Diagnosis and Management of Primary Hypothyroidism”.i The College's statement was originally published on 19 November 2008 and was revised on 14 June 2011. The RCP subsequently endorsed the British Thyroid Association's position statement issued in June 2015 which reviewed more recent evidence.
The British Thyroid Association is “a non-profit learned society of professional clinical specialist doctors and scientists in the United Kingdom who manage patients with thyroid disease and/or are researching into the thyroid and its diseases in humans”.ii The British Thyroid Association was represented at the roundtable on the petition by Professor Graham Williams who noted:
Our understanding of this area is right at the cutting edge of basic science, both internationally and in the UK. We are talking about an area in which knowledge is progressing quite rapidly but on an experimental basis...iii
Figure 2: Professor Graham Williams speaks during the round-table event on 1 October 2013  Scottish Parliament
Scottish Parliament
On 25 June 2015, the British Thyroid Association produced a position statement on the “Management of Primary Hypothyroidism”. The opening lines of the position statement notes:
The management of primary hypothyroidism with levothyroxine is simple, effective and safe, and most patients report improved well-being on initiation of treatment. However, a proportion of individuals continue to suffer with symptoms despite achieving adequate biochemical correction. The management of such individuals has been the subject of controversy and of considerable public interest.iv
The position statement was developed following consultation with members of the British Thyroid Association and relevant stakeholders, including the British Thyroid Foundation – a patient support charity for people with thyroid disorders.iv The British Thyroid Association's position statement notes in relation to patient engagement:
It is important that high-quality, unbiased, evidence-based information about hypothyroidism is made available to patients and the public. We recognise the need to engage with patients and promote more research in hypothyroidism.iv
The British Thyroid Association therefore produced a plain English summary of the guidelines for patients and interested members of the public, which was published on its own website, as well as the British Thyroid Foundation's website.iv
The position statement includes comparative recommendations from both the European Thyroid Association and the American Thyroid Association. Within the UK, the British Thyroid Association's position statement has been endorsed by the Association for Clinical Biochemistry and Laboratory Medicine, the British Thyroid Foundation, the Royal College of Physicians and the Society for Endocrinology. It would appear therefore that professional guidance to clinicians is relatively uniform in the UK, Europe and the United States.
UK and Scottish health authority guidance
The Committee also investigated the guidance available to clinicians in Scotland and the UK from national health authorities. The National Institute for Clinical Excellence (NICE) has produced a clinical knowledge summary for hypothyroidism.i Clinical knowledge summaries provide information on current evidence for primary care professionals. This NICE summary was revised in April 2016 and discusses a number of key issues including ‘diagnosis, management, prescribing, misleading thyroid function tests, assessment of symptoms and other possible conditions’. It is in line with – and makes reference to – the British Thyroid Association's position statement and the Royal College of Physicians’ most recent guidelines.
There is no Scottish Inter-collegiate Guidelines Network (SIGN) guideline on thyroid and adrenal treatment, diagnosis and testing. This issue was explored with SIGN and the Committee agreed to forward the evidence it had received to SIGN, including patient testimonies. The Committee also invited SIGN to work with the lead petitioner in the area of diagnosis and treatment of thyroid conditions and to formulate the necessary questions for guideline development.
SIGN approached the Royal College of General Practitioners (RCGP) to contribute to the development of a document for general practitioners that would “highlight the areas which are missed for patients with remaining symptoms on Levothyroxine, the so called 5% - 10% of patients.” ii RCGP advised that it was not able to participate in this work and that general practitioners could refer to existing NICE guidance.iii SIGN advised that it was not able to take forward a proposal on thyroid testing and treatment.iv
Comment on the role of guidance
Dr Antony Toft, a clinician working in private practice who gave evidence to the Committee at its roundtable session, subsequently wrote an article, published in the Journal of the Royal College of Physicians Edinburgh in December 2017i, in which he commented on the role played by guidelines. The article was highlighted to the Committee by the petitioner.ii Dr Toft was invited to make a submission in light of his article but indicated that he had nothing further to add to the views set out in his article.
In the article, Dr Toft outlined his view that—
“Simply because no two patients present in the same manner, guidelines, by their very nature, are the antithesis of the art of medicine. We cannot afford to underestimate the level of frustration among patients, exasperated by the ‘one solution fits all’ philosophy.”
Dr Toft goes on to state—
“Experience of managing more patients with thyroid disease than most over a period of some 40 years is being trumped by inflexible guidelines; truly a remarkable state of affairs. Others hide behind guidelines to avoid the cost of prescribing liothyronine, which in the UK is exorbitantly priced by the sole supplier at some £250 for two month’s supply of 10 μg daily, when well-travelled patients can obtain supplies for a few euros in Italy and Greece and beyond.”
In respect of current guidelines, Dr Toft’s view is that—
“The facts of the matter are that the current guidelines for LT4 replacement therapy in primary hypothyroidism are not fit for purpose and the continued reluctance to approve additional treatment with liothyronine denies the patient the precision medicine which we are encouraged to adopt, and which many patients crave.”
Professional training and development for general practitioners
The Committee was also interested to understand what training general practitioners receive on the diagnosis and treatment of hypothyroidism. The General Medical Council explained the relevant training framework for medical students in its submission to the Committee dated 4 March 2013:
We set standards and requirements that must be met on the ground; and check that they really are met, through quality assurance activity. Looking specifically at the areas of thyroid and adrenal testing the curricula that include this are General Practice, Endocrinology and Diabetes Mellitus and Otolaryngology (ENT). Each of these curricula…outline the skills doctors in training need to demonstrate…The GMC is satisfied that the submissions from the respective Royal Colleges in relation to the content and requirements of the curricula fulfil our standards and through our quality assurance processes we are satisfied that these are delivered.i
The General Medical Council also noted that continuing professional development courses are provided for qualified general practitioners. This includes a course provided by the Royal College of General Practitioners on “metabolic problems – endocrine and metabolic problems”.ii
In evidence to the Committee on 9 February 2016, Professor Leese, the Specialty Adviser on endocrinology to the Scottish Government's Chief Scientist Officer, noted: “There needs to be increased general awareness of the guidelines. That comes down to educating GPs and advertising the guidelines.”iii
Figure 3: Overview of developments in professional guidance and public information since petition was lodged 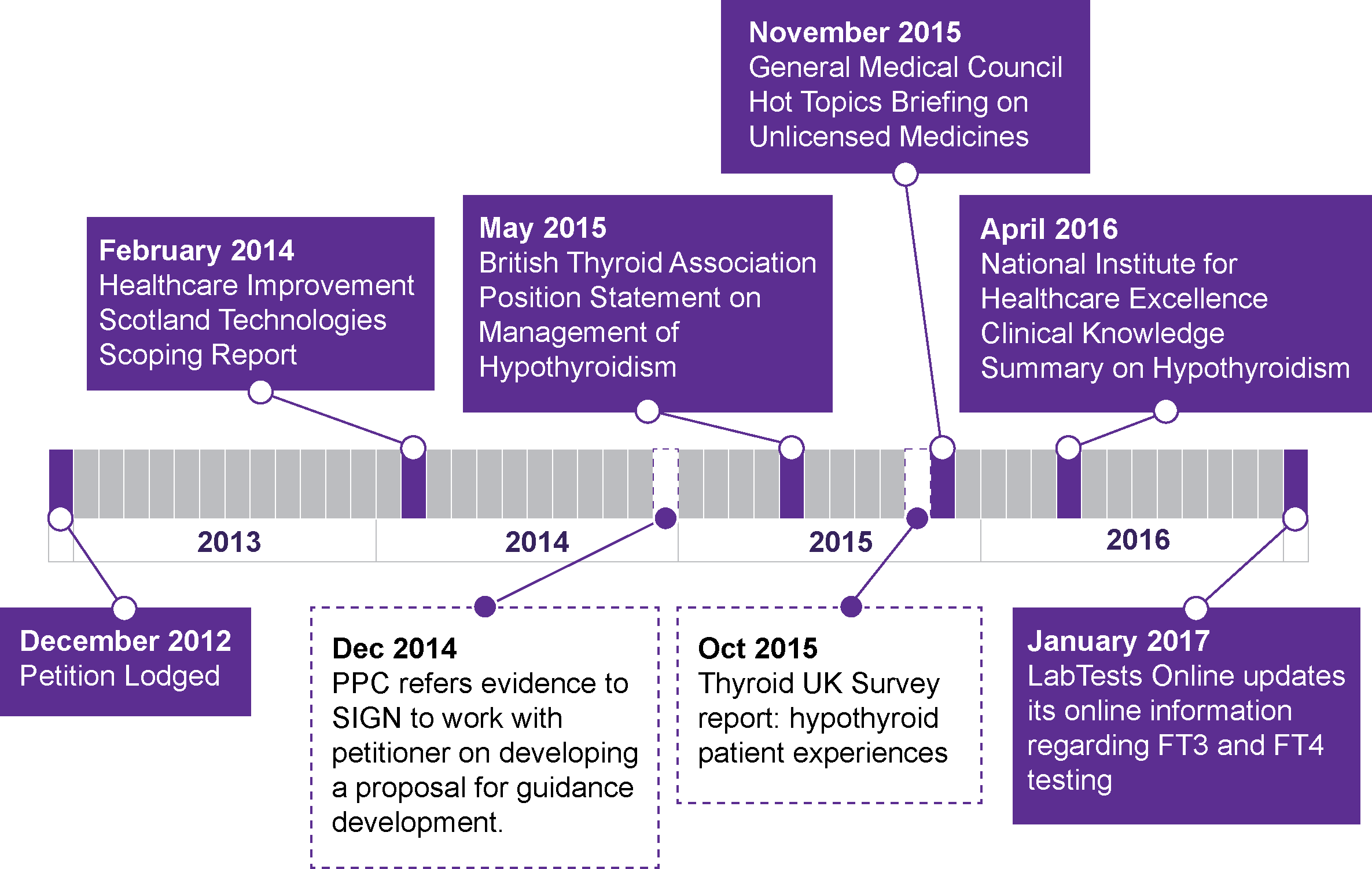
Development of new guideline
The Committee understands that work is currently underway to develop a new NICE guidelinei on the assessment and management of thyroid disease and that this work is intended to be completed by 2019. The Committee notes that the final scope of the review states that the key areas which will be examined include the management of primary hypothyroidism and the management of subclinical thyroid dysfunction. In respect of primary hypothyroidism, issues that will be looked at include both treatment and monitoring, with treatment options to be considered including “levothyroxine [L-T4]; liothyronine [L-T3]; combination of L-T4 and L-T3; thyroid extract; iodine and selenium supplementation.”
In respect of subclinical thyroid dysfunction, the scope includes “treating subclinical hypothyroidism, treating subclinical thyrotoxicosis and monitoring subclinical thyroid dysfunction.”
Conclusions and recommendations
It is clear to the Committee that clinicians have an important, independent role in diagnosing and treating patients. In carrying out this duty, clinicians are guided by evidence-based guidelines. These guidelines are developed by independent experts (health authorities and clinical bodies) and are informed by the available peer-reviewed research on patient treatment.
The Committee welcomes the fact that the profile-raising brought about by the petition appears to have been influential in key sources of clinical guidance being produced or updated by professional bodies and national health authorities.
The British Thyroid Association's acknowledgement that some patients continue to experience ill-heath on standard treatment is a positive step forward in bringing this issue to the attention of clinicians and mainstream endocrinology. The Committee welcomes the British Thyroid Association's steps in consulting with a patient support charity in the process of developing its position statement.
The Committee welcomes the work that is underway to develop a new guideline for thyroid disease and is encouraged to see that the final scope of that work includes a number of the issues of concern that have been raised, both by the petitioners and others who took the time to share their experiences. The Committee will provide a copy of this report to NICE for the information of the committee responsible for developing the new guideline.
The Committee considers it essential that professional bodies continue to work closely with patient support groups and individuals, such as the lead petitioner, so that greater awareness of guidance and patient experience is promoted.
The Committee recommends that the Scottish Government ensures bodies within Healthcare Improvement Scotland also contribute to this work and that, in responding to this report, provides an outline of how that contribution can be made.
Diagnosis and testing
A key concern of the petitioners is the recommended method of diagnosing and testing hypothyroid patients who do not respond well to standard treatment. Existing guidance recommends that where a patient is not satisfied with their response to standard treatment, they “…should be thoroughly evaluated for other potentially modifiable conditions”.i In the petitioners’ view, the patient should still be investigated for potential hypothyroidism using a number of alternative tests. The Committee's scrutiny of diagnosis and testing for hypothyroidism focused on three key issues: the clarity and consistency of public information available online regarding testing; the consistency of testing practices across Scotland; and whether testing for T3 should be routinely conducted.
Initial diagnostic testing
According to NICE and the RCP, the starting point for diagnosing a patient with suspected hypothyroidism is to arrange blood tests for thyroid stimulating hormone and free thyroxine (FT4).i This is used to inform the clinical picture of presenting symptoms in the patient.
Lyn Mynott, Chief Executive of Thyroid UK, raised concerns about the consistency of testing practices during the roundtable session on the petition:
Part of the problem is that in some areas only a thyroid stimulating hormone test is done, whereas in others a free T4 test is done as well. It is very rare to get a free T3 test done. That needs to change, as the practice is not consistent throughout the United Kingdom.ii
In light of these concerns, the Committee reviewed what public information is available to patients about routine testing procedures. The Committee noted that the Lab Tests Online and NHS Inform websites explain that in some cases, a clinician may decide to arrange for FT3 (or total T3) testing in addition to the TSH and FT4 testsiii.
The Committee observed, however, that the information provided by Lab Tests Online made no distinction between FT3 and TT3 and FT4 and TT4 testing. This appeared to contradict information on the NHS Inform and NHS Choices websites. Parliament officials sought clarification on this point on the Committee's behalf. Following this enquiry, the information was revised to conform to information on NHS Choices and NHS Inform to clarify the difference in testing and when they might be used.
The petitioners’ view is that testing for FT3 or total T3 should be done as a matter of course, not only at the initial diagnosis stage, but when treatment with the standard treatment (T4) is ongoing and symptoms persist. In this regard, one of the petitioners, Sandra Whyte, commented in evidence to the Committee: “The tests are out there, but they are not on the NHS. We had to get private testing done, and that showed it up straight away.”ii
The petitioners’ call for T3 testing to be conducted routinely was countered by Dr Toft who explained:
On the request that T3 should always be checked, I am sure that my colleagues will agree that, in attempting to diagnose an underactive thyroid gland, T3 is the least useful test, in our experience. It is often misleading – it is often normal in patients with profound hypothyroidism, for example. That is why it is not offered as standard by laboratories for the investigation of possible underactivity of the thyroid.ii
Professor Graham Williams, President of the British Thyroid Association, who also attended the round table agreed with Dr Toft, noting:
It is very misleading to be thinking about measuring T3. I agree with Dr Toft that the optimal measurement for patients who are taking T4 and who have hypothyroidism should be the TSH and the T4. I do not think that there is a place at the moment for T3 because we do not understand enough about it.ii
Ongoing treatment review and management
The British Thyroid Association's position statement notes that after treatment has been initiated, the patient's TSH should be monitored 6-8 weekly until it is stabilised.i This further testing and monitoring will inform the clinician whether the patient's medication needs to be altered to achieve a stable TSH. If a patient's TSH concentration is abnormal, this test will usually be followed up with an FT4 measurement (or occasionally total T4).ii
The Committee received written and oral evidence from Dr John Midgley, a researcher in biochemistry who co-patented the FT4 and FT3 tests. Dr Midgley explained in written evidence that the common experience of many patients is that “...TSH is almost always the only test carried out in treatment control, free T4 occasionally, and free T3 almost never.”iii
Dr Midgley explained in oral evidence:
It is unfortunate that the test for thyroid stimulating hormone that I mentioned is now overreaching. It is supposed to be successful in diagnosing the onset of disease through testing for thyroid underfunction or overfunction—for which it is perfectly suitable—as well as for the control of treatment, for which it is totally unsuitable. Extending its use to a function for which it is unsuitable has led to a significant number of patients being wrongly diagnosed, wrongly treated or not treated at all.iv
In Dr Midgley's view, the “…free T3 is the most accurate assessment of control of the response to treatment (be it T4 monotherapy, T4/T3 combination or T3 alone)”.iii Dr Midgley considered that “the use of TSH is inaccurate and frequently misleading, with unpleasant consequences for the patient.” iii
Dr Toft later clarified that, whilst he does not support routine T3 testing: “There is no reason why T3 should not, from time to time, be measured in patients who are being treated for an underactive thyroid”.vii
Following its survey of patient experience, Thyroid UK made a recommendation to the Scottish Government that full thyroid function tests, including TSH, Free T3, Free T4 and thyroid antibodies (TPO and TgAb), should be conducted for patients with symptoms of hypothyroidism or hyperthyroidism. In its view, this “…would ensure prompt and accurate diagnosis and fewer erroneous diagnoses”.viii
The Scottish Government's response to this conclusion was outlined in a written submission to the Committee:
This range of tests is sometimes, but not always, needed. If the diagnosis and treatment is clear then these extra tests may not be needed, but if there is uncertainty they may be. Testing is thus personalised to the individual, which is less costly to the NHS than using a battery of tests for all individuals. The tests required for an underactive thyroid are different to an overactive thyroid. This is therefore an individual clinical decision in each case.ix
Biochemists’ role in diagnosis
The usual practice for testing will be for a clinician to take a blood sample from the patient and send the sample to a laboratory for testing.i The Committee was informed that the testing process and how it is being used to inform diagnosis is causing patients considerable frustration.ii Dr Midgley's view is that:
At the moment, the chemistry dominates the presentation of symptoms by the patient. That is the wrong way round. The presentation of symptoms by the patient should dominate over biochemical parameters, which should be suggestions and indicators but not dictators of the situation. That is very important.ii
Thyroid UK chief executive, Lyn Mynott, stated during the roundtable discussion:
We hear all the time that GPs are asking for T3 testing, but the biochemists at the local laboratory will not provide it, sometimes because – they say – the TSH is within the range, but sometimes they give no reason. Often, doctors ask for the T3 test, but the biochemist seems to have priority over the GP who is treating the patient, although the biochemist has not seen the patient or the patient's notes. ii
Ms Mynott's statement appeared to be supported by the findings of Thyroid UK's patient experience survey in 2015. The survey asked patients to comment on their diagnostic and treatment pathways and whether they had been refused testing by their GP, endocrinologist or local laboratory. The survey methodology explains that 4,299 respondents completed the survey. It does not provide a breakdown of the respondents, including how many respondents were treated in Scotland.v The results showed:
Between 1% and 14% of the time laboratories did not undertake the test despite it being ordered by an NHS GP. Failure to undertake FT4 and FT3 tests were the highest at 10% and 14% respectively;
NHS GPs stated that the laboratories would not undertake the tests to 22% of respondents for FT4 tests, 29% for FT3 test and 29% for reverse T3 (rT3) tests;
Laboratories did undertake tests for TSH, RT3 and DIO2 for NHS endocrinologists but failed to undertake tests ordered by them 10% of the time for FT3; 3% for FT4 and 2% for TPO and TgAb;
Endocrinologists stated that laboratories would not undertake testing to 4% - 23% of respondents with RT3, FT3 and FT4 at 23%, 17% and 12% respectively.v
In response to these results, Thyroid UK made a recommendation that laboratories should not refuse testing requested by a clinician.v
The Scottish Government responded to the survey results, noting:
Laboratories usually undertake what is requested of them, but sometimes it is helpful for them to guide clinicians, as they are experts in the field of testing, and can help as to what extra tests may be useful and what tests may not be. Laboratory experts may help clinicians identify when abnormal thyroid tests are not due to thyroid disease and when results are problematic.viii
Commenting on the Scottish Government's response on this issue, Dr Midgley expressed his view that laboratories have “unacceptable sweeping powers” in patient testing and diagnosis. He explained further:
The clinical chemist has not seen the patient and has no knowledge of their detailed presentation to the doctor. This attitude has reduced the patient's diagnosis to determining it by an anonymous set of numbers which are either in or out of the respective normal ranges, which themselves are uncertain and vary significantly from laboratory to laboratory…Medical practice must reconsider the importance of presentation of the patients themselves as opposed to statistical assignments made entirely from an inadequate number of tests and dictation to the doctor by those not intimately concerned with the patient.ix
Reference ranges
In the course of forming a diagnosis, clinicians and laboratory biochemists rely on “typical reference ranges” for “normal” thyroids to interpret the results from blood tests.i These ranges are only a guide and will often vary according to laboratory because different tests or methodologies might be used.i In this regard, the British Thyroid Association's position statement also acknowledges: “The reference range varies in different ethnic communities, pregnancy and by age”.iii
The British Thyroid Foundation has produced the following table,i which indicates the “typical reference ranges for normal thyroids”:
Figure 4: British Thyroid Foundation typical reference ranges for normal thyroids
Test From To Units TSH 0.4 4/4.5 Milliunits per litre (mU/L) FT4 9.0 25 Picomoles per litre (pmol/L) FT3 3.5 7.8 Picomoles per litre (pmol/L)
Lab Tests Online explains how the reference ranges are calculated:
A large number of individuals from a group, who are thought to represent a "normal" population, will be tested for a particular laboratory test. The reference range is then derived mathematically by taking the average value for the group and allowing for natural variation around that value (plus or minus 2 standard deviations from the average). In this way, ranges quoted by labs will represent the values found in 95% of individuals in the chosen ‘reference’ group. In other words, even in a "normal" population, a test result will lie outside the reference range in 5% of cases (1 in 20). This is why the term "reference range" is preferred over "normal range".v
Lab Tests Online also note caution on the use of reference ranges:
For most tests, the reference ranges are specific to the laboratory that produces the test results. Different laboratories use different kinds of equipment and different kinds of testing methods. That means they have to establish their own ranges, and will provide your test result with an accompanying reference range appropriate to the laboratory. The doctor should therefore apply the reference range supplied by the laboratory which performed the test rather than that used by another laboratory or one given in a book.v
Some respondents were critical of the process by which reference ranges are developed and their use in diagnosis. Thyroid Patient Advocacy noted that different ranges are used in other countries and its view is that the range used in the UK should be reviewed.vii
Dr Midgley was also critical of the reference ranges in his written evidence, describing the current approach as committing “the sin of categorisation”.viii In his written evidence, Dr Midgley explained “Modern knowledge shows that the reference range for TSH and free T4 derived from healthy euthyroid subjects is inappropriate for patients under thyroid hormone therapy.”ix Dr Midgley elaborated on this point in oral evidence when he explained:
…it is not sufficient just to put someone in the range; you have got to put them in the range in the position where their optimum health is to be found. Since you do not know what that value was when they were well—because it was never measured—there is a lot of flailing about going on to find out what the best solution is. That is what I mean by the sin of categorisation. It is shoehorning people into the normal range and saying, “That's okay—job done.” That is wrong.viii
The Committee also heard directly from many patients who are strongly dissatisfied with how the reference ranges are used in practice by clinicians. Carolyn Wileman's written submission to the Committee serves as an illustration in this regard:
At present treatment is withheld until a single blood test, the TSH, reaches an arbitrary figure of 10. A truly healthy person would have a TSH of around 1. Mine only ever got to 4 and I was so unwell I could barely stand up, had 47 separate symptoms and was off work (ironically within the NHS) for 4 months. My GP obviously considered I was unfit for work as the sick certificates kept coming, but still refused to give me any treatment at all, either for my obvious hypothyroidism or anything else. On presenting private blood tests to my GP to prove that I had antibody levels 100 times above the reference range, indicating autoimmune thyroiditis, I was reluctantly referred to an endocrinologist (at the very hospital where I work) who also refused me any treatment based solely on my TSH.xi
Dr Toft, commented on how the reference range is interpreted by clinicians during the round table discussion on 1 October 2013: “…the criticism that there might not be an appreciation of the issue is probably fair, but more with regard to general practice than specialist practice”.viii
The British Thyroid Association's position statement noted that “There has been growing controversy about the upper limit of the reference range for serum TSH”.iii Its conclusion in response to calls to amend the reference range is that:
The evidence in favour of narrowing the serum TSH reference range is not convincing and cannot justify the large increase in the number of healthy people that would require investigation.xiv
When asked about the use of blood tests and reference ranges as diagnostic tools, Professor Leese, Chief Medical Officer Speciality Adviser, commented in evidence to the Committee on 9 February 2016:
The British Thyroid Association guidelines…suggest – this is nothing new – that the blood tests are not the whole picture. Placing someone on thyroxine involves a balance between treating symptoms and treating the blood tests. The blood tests give a range that a person should be within in terms of safety. Within that, the dose of thyroxine can be moved around, as long as the figures are kept within the safe range. That is perhaps more explicit in the document, but that has always been the case.viii
The Royal College of Physicians notes in its guidance: “We recognise that different methods used for testing blood can give differing results, and we support the international initiative for greater harmonisation of reference ranges and of the units used in expressing results.”xvi
Opportunities for shared learning and harmonisation
The Committee understands that staff working in clinical biochemistry laboratories in all 14 regional NHS Scotland Health Boards are represented at the Scottish Clinical Biochemistry Managed Diagnostic Network (‘the Network’).
This Network, which is hosted by the NHS National Services Division Scotland, has a lead role in promoting best practice, harmonisation and improving the evidence-base for diagnostic testing in Scotland. The Committee noted in its evidence gathering that the Network leads on a Demand Optimisation project, which is dedicated to ensuring that patients receive “the right tests at the right time in the right way”. In this regard, the Committee understands that the Network has not produced any material on thyroid testing protocols in recent years.
The Scottish Government contacted the Network in 2013 to:
“…further inform our understanding of the issues with TSH only testing. We are also seeking advice on the appropriateness of the current range of results which are ‘normal’ and insight into the role of laboratory staff in interpreting test results”.i
The Scottish Government advised the Committee in 2014 that the Network's response indicated that “there is no evidence base to support the changes being sought by the petition".ii
The Committee did not receive a copy of the Network's correspondence and as such its reasons for forming this view are not clear. The totality of evidence presented to the Committee over the course of its consideration of the petition appears to suggest that the consistency of diagnostic testing for hypothyroidism and its role in diagnosis merits further investigation. In addition, there may be benefit for the Network to work with other professional bodies, such as the Royal Colleges, to clarify the working relationship between laboratory biochemists and clinicians in diagnosing and treating patients.
Conclusions and recommendations
The Committee recognises the importance of accurate and accessible public information for patients to inform their treatment decisions and options. In this regard, the Committee is pleased that its work has resulted in updated public information regarding different testing options being made available on Lab Tests Online.
The Committee recommends that the Scottish Government brings the evidence received on the variation in thyroid testing in Scotland to the attention of the Scottish Clinical Biochemistry Managed Diagnostic Network. In doing so, the Committee suggests that consideration should be given to moving towards the development of a single, national protocol for testing, along with an accompanying process for issuing consistent advice to primary care practitioners for use when considering and interpreting diagnostic tests for suspected hypothyroidism.
Treatment
According to the British Thyroid Association, the standard treatment for hypothyroidism is synthetic T4 (levothyroxine).i The British Thyroid Association's position statement recommends that patients should be reviewed at regular intervals until a stable TSH level is achieved with the treatment. A patient's symptoms and blood test results inform the clinician whether any adjustments to the treatment medication are required. The standard treatment appears to work for most people because they can naturally metabolise T4 into T3, the active form of the hormone. The lead petitioner argues, however, that some patients are not able to naturally metabolise T4 into T3 and continue to experience symptoms despite receiving the standard treatment (T4).ii The British Thyroid Association's position statement notes in this regard:
It is acknowledged that a proportion of individuals on L-T4 [T4] are not satisfied with therapy and have persistent symptoms despite a normal serum TSH. Such symptoms should be given due consideration and patients should be thoroughly evaluated for other potential modifiable conditions.i
The petitioners, on the other hand, contend that such patients should have access to alternative treatments, such as T3 or natural desiccated thyroid. In this regard, Lorraine Cleaver expressed her frustration at the current treatment options available to patients on the NHS:
I will finish by saying that there are 82 medicines for type 2 diabetes available on the NHS list, 47 for depression, 45 for acne, 16 for athlete's foot, three for hiccups, three for dandruff and one for thyroid – there is something very wrongiv.
Ms Cleaver and other patients presented evidence to the Committee that suggested some patients are self-medicating using alternative medications purchased online, such as natural desiccated thyroid. Patients appear to be taking this action because their clinicians are not open to prescribing alternative treatments.v
Natural desiccated thyroid
Ms Cleaver considers that natural desiccated thyroid should be made available to patients on the NHS. In her view, this medication can be an effective alternative for patients who do not respond well to the standard treatment (T4) because their bodies are not able to convert this hormone into its active form (T3).i
Natural desiccated thyroid is a medication that is extracted from porcine thyroid glands.ii Until the middle of the twentieth century, when synthetic levothyroxine was introduced, natural thyroid extract was the standard treatment for hypothyroidism.iii The Committee heard evidence that its use was supported by professionals at the time because treatment options for patients were limited due to the absence of synthetic medications.ii Once synthetic hormone medications were developed, they became the standard treatment for hypothyroidism and natural desiccated thyroid was removed from the National Formulary.
Natural desiccated thyroid is not currently a licensed medication in the UK. According to Thyroid UK, natural desiccated thyroid is manufactured by Forest Pharmaceuticals, RLC Labs and Erfa Canada Incorporated.v The Committee understands that natural desiccated thyroid is stocked by a number of wholesalers in the UK and can be purchased online.vi
Whilst not a licensed medication, natural desiccated thyroid can be prescribed by clinicians to patients on a “named patient basis”. This exemption is set out under regulation 167 of the Human Medicines Regulations 2012.vii According to this exemption, an unlicensed medicinal product may only be supplied in order to meet the special needs of an individual patient.viii The MHRA's guidance explains: “An unlicensed medicinal product should not be supplied where an equivalent licensed medicinal product can meet the special needs of the patient”.viii
Professional bodies and health authorities in the UK do not currently support the use of natural desiccated thyroid as a treatment for hypothyroidism. The British Thyroid Association's position statement notes in this regard: “There is no convincing evidence to support routine use of thyroid extracts…in the management of hypothyroidism”.x The Royal College of Physicians does not recommend its use as a treatment for hypothyroidism and cites concerns about the amount of T3 in some products.xi
UK institutions, such as NICE, also do not currently recommend the use of natural desiccated thyroid as a treatment for hypothyroidism.xii Healthcare Improvement Scotland's Technology Scoping Report noted the only relevant study identified comparing natural desiccated thyroid with T4 found no evidence of a difference in symptoms, general wellbeing and cognitive function.xiii
The concerns of mainstream endocrinology appear to relate not only to effectiveness, but also safety. xiv The European Thyroid Association's position statement notes in this regard:
All these combination tablets (including those containing animal thyroid extract) are potentially harmful as due to their relatively high T3 content they carry a risk of inducing symptoms of thyroid hormone excess.xv
Professor Williams, President of the British Thyroid Association, explained in more detail the general concerns about natural desiccated thyroid as a treatment option in the round table session:
Thyroid extract is produced from pigs…and the ratio is of T4 to T3 in the pig thyroid is approximately 4:1 or 5:1, whereas in the human thyroid the ratio of T4 or T3 is about 14:1 or 15:1. The constituents that you are starting with are different from those in humans, which makes it pretty difficult to manage the dosage and what is being done with it. That is one reason why there has been a lot of debate and discussion in the area.ii
The Committee also received written evidence from Dr Midgley who supports the use of natural desiccated thyroid in treatment. Dr Midgley called for an “open-minded” approach to treating and diagnosing patients with suspected hypothyroidism.xvii In his view, the regulation of natural desiccated thyroid in the US is evidence that the product is safe to use and its hormone contents can be adequately controlled.xvii The petitioners also argue its use up until synthetic products became available is also evidence in support of its safety and effectiveness as a treatment option.xix
Maureen Watt MSP, then Minister for Public Health, responded to concerns about the use of natural desiccated thyroid in mainstream endocrinology in evidence to the Committee on 9 February 2016: “I do not think that I, as a politician, should say what the medical profession should do. That is up to the medical profession.”ii In this regard, the Minister expressed the view that the British Thyroid Association's guidelines “…reflect current best practice in the management of hypothyroidism”.ii
Liothyronine (T3)
Liothyronine, or T3, is the active form of the thyroid hormone levothyroxine (T4). It is produced synthetically in the UK under licence. Until summer 2017, the only licence for T3 in the UK was held by a manufacturer, Amdipharm Mercury Company Ltd.i
The lead petitioner considers that hypothyroid patients who do not respond well to T4 should be trialled on T3. Ms Cleaver explained her personal experience to the Committee in this regard:
I would not even be sitting here talking to the committee if I had not been able to push my case with my general practitioner, even though I was very ill at the time, to get to the right consultant who was willing to try me out on triiodothyronine—T3—and basically bring me back to life.ii
Figure 5: Lorraine Cleaver provides evidence to the Committee on 1 December 2015  Source: Scottish Parliament
Source: Scottish Parliament
The then Minister for Public Health provided some context to the Committee on the use of this treatment option in Scotland, noting: “there are about 6,500 prescriptions in Scotland for T3. That involves 1 per cent of people who are on thyroid medication”.ii
Existing clinical guidance
The British Thyroid Association commented during the roundtable event in 2013: “I don't think there is a place at the moment for T3 because we do not understand enough about it.”ii The British Thyroid Association later published its position statement, which reviewed the position of other professional bodies on the use of T3 as a treatment option for hypothyroidism:
Both guidelines [European Thyroid Association and American Thyroid Association] strongly recommend that L-T4 [T4] remains the therapy of choice in hypothyroidism and do not support the routine use of L-T4 [T4]/L-T3 [T3] combination therapy due to insufficient evidence from controlled trials, lack of long-term L-T3 [T3] safety data, and unavailability of L-T3 [T3] formulations that mirror natural physiology.v
The British Thyroid Association's position statement notes that “Although some nonmainstream practitioners advocate the use of alternative thyroid therapies including liothyronine (L-T3) and thyroid extracts, combination therapy with L-T4 [T4] and L-T3 [T3] is also prescribed by accredited specialists.”v The British Thyroid Association concludes, however, that: “serum T3 should not be used as a therapeutic target in the management of hypothyroidism as the value of this approach is unproven”.v
On the issue of combining the standard treatment (T4) with T3, the British Thyroid Association's position statement explains that this may be merited in some circumstances:
L-T4 [T4]/L-T3 [T3] combination therapy in patients with hypothyroidism should not be used routinely, as there is insufficient evidence to show that combination therapy is superior to L-T4 [T4] monotherapy…If a decision is made to embark on a trial of L-T4 [T4]/L-T3 [T3] combination therapy in patients who have unambiguously not benefited from L-T4 [T4], then this should be reached following an open and balanced discussion of the uncertain benefits, likely risks of over-replacement and lack of long-term safety data.v
The then Minister for Public Health indicated to the Committee that the Scottish Government's role is to “…provide policies, frameworks and resources to national health service boards to allow them to deliver services that meet the needs of their local populations”.ii In this regard, the Scottish Government's view on T3 was the same as natural desiccated thyroid, insofar as it considers that the British Thyroid Association's position statement reflects “current best practice in the management of primary hypothyroidism”.ii
Treatment costs and production
The lead petitioner has raised concerns that clinicians may be reluctant to prescribe T3 to patients due to its high cost. Ms Cleaver explained in evidence on 1 December 2015, that there is only one manufacturer of T3 in the UK who charges “£100 for 28 tablets…which is available for £2 in Europe”.xi
In response to concerns about the cost and supply of T3, the Committee invited the then Cabinet Secretary for Health and Wellbeing, Alex Neil MSP, to give evidence on 25 June 2013. Mr Neil explained the likely causes for these issues: “We need to bear in mind the relatively small size of the market, the very expensive raw material and the expensive and complicated manufacturing process for the drug”.ii
The Committee raised these concerns with the Scottish Government again in an evidence session on 9 February 2016. Alpana Mair, Deputy Chief Pharmaceutical Officer, noted that the number of T3 prescriptions in Scotland is small in comparison to T4 prescriptions. In this regard, Ms Mair observed that commercial factors would influence another company's decision to apply for a licence to produce T3 in the UK and the Scottish Government is not aware of any other companies seeking to do so.ii
In November 2017, the Competition and Markets Authority (CMA) announced that, following an investigation, it had made a provisional finding that the pharmaceutical company, Concordia, had been overcharging the NHS for T3. The CMA noted that the the price the NHS "paid per pack rose from around £4.46 before it was de-branded in 2007 to £258.19 by July 2017, an increase of almost 6,000%, while production costs remained broadly stable."
The CMA's findings have been set out to the pharmaceutical company in a Statement of Objections. The CMA explains that a Statement of Objections "gives parties notice of a proposed infringement decision under the competition law prohibitions in the Competition Act 1998 and the EU law equivalents. It is a provisional decision only and does not necessarily lead to an infringement decision. Parties have the opportunity to make written and oral representations on the matters set out in the Statement of Objections. Any such representations will be considered by the CMA before any final decision is made."
The lead petitioner explained to the Committee that the fact that a single manufacturer produces licensed T3 in the UK has resulted in supply issues. Ms Cleaver noted in this regard: “because one company makes the drug, it has to recalibrate all its machinery when it does a manufacture run. It forecasts what it will need and if that does not meet the need, it will be another several months before it is ready to recalibrate and make another batch.”ii
The Committee received a submission from the manufacturer, which explained that “disruptions to supply of liothyronine can occur if one large batch is rejected at the final manufacturing stage. This can be due to any number of reasons and the product cannot be remanufactured immediately until all the pending issues are rectified.”xv The manufacturer could not guarantee that disruptions would not occur again but assured the Committee that it takes its manufacturing duties very seriously.
The Medicines and Healthcare Products Regulatory Agency (MHRA) provided a submission to explain what action it was taking to address the supply issues. It noted:
In the case of Liothyronine, in the interim, supplies of unlicensed alternatives to this medicine were imported from abroad to avoid shortage in the UK. The MHRA wrote to healthcare professionals on 21 May 2013 to explain the situation and to provide clinical advice on the use of imported alternatives. A communication was also published on the MHRA’s website…xvi
The MHRA noted that whilst it would be beneficial in terms of the security of supply of T3 in the UK to have more than one licensed manufacturer, the MHRA “is not in a position to hold or solicit for new product licences themselves”. xvi For this reason, the only way to increase the number of manufacturers in the UK would be for a manufacturer to apply for a licence from the MHRA.
Alpana Mair explained what action the Scottish Government has taken in relation to the supply of T3 in recent years:
Our Scottish officials are in regular contact with colleagues in the Department for Health, who will work with the manufacturers on improving their processes to ensure that there is continuity of supply for patients and that we do not experience the same problems that arose a couple of years back. Work has been undertaken to try to resolve some of those issues, and there were no supply issues at all in 2015.ii
Two additional licences for T3 have now been granted, one of which is for a lactose-free version of the medication.
Doctors’ discretion to prescribe alternative treatments
The Committee also considered doctors’ discretion to prescribe alternative treatments and the factors that may influence their decision to do so. The lead petitioner, Lorraine Cleaver, explained in evidence to the Committee that, in her experience, doctors are under pressure from professional bodies not to prescribe unlicensed medication: “My new problem is that the General Medical Council is intent on deregistering the doctor who saved my life.”i
In the Committee's roundtable discussion, Lyn Mynott, Chief Executive of Thyroid UK, commented:
I do not know of any NHS doctors being taken to the GMC for treating patients with Armour Thyroid [a natural desiccated thyroid product], but we are afraid that this will happen. Patients regularly phone us to say that they have asked for a trial of T3 or Armour and their doctor has told them “I am sorry – I will get into trouble if I prescribe you this medication. I can't do it”. That is where the conversation finishes.i
The British Thyroid Association's position statement makes reference to a 2006 paper that cited figures from the US showing that 3.6% of endocrinologists reported that they would prescribe T3 to patients with hypothyroidism with persistent symptoms and normal biochemical thyroid status.iii Figures for Scottish or British endocrinologists were not provided. In the context of its recommendations on the prescription of T3, the British Thyroid Association's position statement notes that:
Clinicians have an ethical responsibility to adhere to the highest professional standards of good medical practice rooted in sound evidence. This includes not prescribing potentially harmful therapies without proven advantages over existing treatments.iii
Tara Wilmott, representing the GMC’s Education and Standards Directorate, commented in the roundtable: “…our guidance to doctors is that they need to follow the published standards and that working outwith those standards and guidelines could cause them to get into difficulties.”i
Dr Anthony Toft commented n the round table on why certain clinicians had been investigated by the GMC for prescribing natural desiccated thyroid: “Those individuals prescribed thyroid extract without doing any thyroid blood test to begin with in order to prove the diagnosis. They also did not record any blood test results after prescribing thyroid extract, and they were giving very high doses of thyroid extract.”i
Figure 6: Round table - 1 October 2013  Source: Scottish Parliament
Source: Scottish Parliament
In November 2015, the GMC published an online briefing entitled “Hot topic: prescribing unlicensed medicines”.vii The briefing explains whether clinicians would be risking their registration to practice if they were to prescribe an unlicensed medicine. The briefing notes the following:
Doctors are often worried about prescribing unlicensed medicines as we say that they must take responsibility for the prescription, but of course we expect this whether the medicine is licensed or not. You are responsible for all prescriptions you sign and your decisions and actions when supplying and administering medicines and devices (or when they authorise or instruct others to do so).
We expect you to carefully consider any treatment that you prescribe, and we expect you to be able to justify your decisions and actions when prescribing, administering and managing medicines regardless of whether they are licensed or unlicensed.
Importantly, prescribing unlicensed medicines will not put your registration at risk any more than other areas of practice covered by our guidance.
The GMC's briefing also includes information on what factors a clinician should take into account when prescribing an unlicensed medicine. The briefing explains: “You should be satisfied that there is sufficient evidence or experience of using the medicine to demonstrate its safety and efficacy.” viii
Conclusions and recommendations
The Committee recognises the Government's role in relation to treatment is to provide policies, frameworks and resources to National Health Service boards to allow them to deliver services that meet the needs of their local populations. In doing so, it is vital that consideration is given to clinical evidence, knowledge and guidance such as that offered by bodies, such as the British Thyroid Association or NICE.
The Committee considers that the clinical information referred to in this report now makes clear the treatment options that can be considered by doctors and patients, as well as clarifying the position on the prescription of unlicensed treatment options, such as natural desiccated thyroid.
The Committee recognises the concerns that the petitioner has raised about the issue of supply of T3. The Committee notes that the licensing of medication is reserved but that the Government's action has included discussing previous supply issues with the UK Department for Health to monitor the supply of T3 in the UK. It understands that the issue of temporarily sourcing unlicensed supplies of the medication have since been addressed.
In relation to the production and costs of T3, the Committee understands that the market for this treatment in the UK is relatively small. For the majority of the period in which this petition has been under consideration, there has been only one licensed supplier of T3. However, the Committee understands that two further licences were granted in summer 2017. The Committee asks the Scottish Government, in its response to this report, to provide information about the impact that the granting of further licences may have on the costs of T3. The Committee asks this particularly with regard to the Competition and Markets Authority's recent announcement of a provisional finding that Concordia has overcharged the NHS by millions for T3.
Finally, on the issue of natural dessicated thyroid, the Committee notes that this is not currently licensed and understands there are different views about its efficacy as a treatment for hypothyroidism. The Committee is not aware of any current applications for natural dessicated thyroid to be licensed but draws the attention of the MHRA to the anecdotal evidence received on this petition and the number of people who report that use of natural dessicated thyroid leads to an improvement in their symptoms.
Research
The Committee understands that clinical guidance on hypothyroidism is evidence based and informed by clinical research.i The petitioners raise concerns about the evidence base for existing clinical guidance in the petition background information. In this regard, they consider clinical studies to date have not adequately investigated the treatment needs of patients who continue to feel unwell on standard treatment. The petitioners’ cite anecdotal evidence from a minority of patients, which they argue contradicts existing recommendations for treatment.i
Clinical studies
How research informs clinical guidance
The then Minister for Public Health, Maureen Watt MSP, explained to the Committee in evidence on 9 February 2016 how clinical trials inform best practice guidance on the management of primary hypothyroidism:
The position statement by the British Thyroid Association, which was published in May 2015, clearly sets out its recommendations on the management of primary hypothyroidism on the basis of the current literature and review of the published positions of the European Thyroid Association and the American Thyroid Association…they therefore reflect current best practice in the management of primary hypothyroidism…i
Professor Leese, who was supporting the Minister at the evidence session, commented on the existing evidence base: “…11 out of 12 trials showed no benefit from liothyronine for patients feeling unwell on thyroxine, and one randomised control trial with desiccated thyroid showed no benefit.”i The Scottish Government provided the Committee with a list of the relevant randomised trials that have been conducted to date on liothyronine (T3).iii
The Minister explained further: “It should be recognised that progress in clinical science has been, and should continue to be, based on properly conducted, scientifically based trials that strive to eliminate any error or unrecognised confounding issues”.i
Calls for further research into alternative treatments for patients
Elaine Smith MSP summed up the concerns of the petitioners’ and wider patient body in evidence to the Committee on 1 December 2015: “To me, the bottom line is that a lot of patients in Scotland are not getting the right treatment…More research needs to be done to show what is happening.”i
There was agreement from clinicians that more research in this area is required. When speaking at the roundtable in 2013, Professor Williams acknowledged: “Our understanding of this area is right at the cutting edge of basic science, both internationally and in the UK.”
Professor Leese agreed that more research could be useful in addressing the treatment of patients who continue to feel unwell on the standard treatment: “That is where we are at the moment, but that will not stop us doing more clinical trials and perhaps trying to look at specific patient groups that might benefit from those drugs”.i
Dr Midgley agreed that further research would be beneficial but raised concerns about whether clinical trialling would be an appropriate research methodology for the relevant patient group:
Such studies could not be carried out validly by randomized clinical trials. For one thing, a significant number of potential subjects have removed themselves from being chosen for any trial, through self-medication, absenting themselves from possible selection. As these are heavily represented by subjects requiring additional T3, any attempt at a clinical trial fairly and randomly selecting a typical patient group under direct medical supervision is immediately invalidated.vii
The Committee investigated which agencies or institutions could play a role in supporting or leading on further research in this area. The Scottish Government advised the Committee that the relevant Scottish Government agency is the Chief Scientist Office (CSO).viii The CSO's funding criteria require research projects to be led by a Scottish-based clinician or scientist and that the research must have the potential to improve the health and wellbeing of the people of Scotland. The Scottish Government explained:
The CSO would welcome applications for research projects aimed at identifying optimum treatment modalities for hypothyroidism. These would go through the same rigorous independent review process as applications in any other clinical area. viii
SPICe also identified a number of other relevant UK research funding bodies, including: the Association of Medical Research Charities; Medical Research Council; the National Institute for Health Research (NIHR), including NIHR's Evaluation, Trials and Studies Coordinating Centre; UK Clinical Research Collaboration; and the UK Research Office.x
Patient experience and anecdotal information
Prior to the petition being lodged, there appeared to be limited studies into the experience of hypothyroid patients who do not respond well to standard treatment. The Committee therefore welcomed the decision by the then Minister for Public Health, Michael Matheson MSP, to conduct a listening exercise in response to the petitioners' concerns.i The stated purpose of the exercise would be “to explore how patients understand and feel about their quest for a diagnosis and/or treatment in areas where the evidence is limited, the science is uncertain or disputed and/or where a condition is rare or obscure, or not widely recognised.”i
The Scottish Government commissioned Thyroid UK to conduct a UK-wide survey of patients and later clarified that this would form a listening exercise. Thyroid UK published a report on “Hypothyroid Patient Experiences” in October 2015 and made a number of recommendations.iii The cost of the survey was £1,064.69.iv
Maureen Watt MSP, then Minister for Public Health, gave evidence to the Committee on 9 February 2016. When asked about the Scottish Government's response to the findings, the Minister explained that the survey was UK-wide and that Scottish patient responses could not be extrapolated from the data.v
The Minister also commented on how the survey design limited the extent to which the Scottish Government could use the results to inform policy or practice:
It did not involve a randomised sample, as one would normally expect in, say, a clinical trial. The people who responded are very involved in the issue and the figures quoted are likely to be an overestimation of the situation.v
The Committee asked the Scottish Government to clarify what action it would be taking to address the issues raised by the survey and whether it intends to implement Thyroid UK's recommendations. The Scottish Government explained that the survey would be published online and at the Committee's request it also provided a written response to each of the Thyroid UK's recommendations.vii
Maureen Watt MSP commented further on how the findings of the survey could be taken forward in evidence to the Committee on 9 February 2016:
It should be recognised that progress in clinical science has been, and should continue to be, based on properly conducted, scientifically based trials that strive to eliminate any error or unrecognised confounding issues. It is appreciated that progress can sometimes be frustratingly slow, but that is the consequence of trying to get things right and ensure patient safety, which is paramount at all times. Anecdote and clinical observation can be useful to raise scientific questions, but such questions need to be tested rigorously; otherwise, the approach can be potentially detrimental and dangerous to patients, as well as wasteful of NHS resources, not just for thyroid disease but for all other medical conditions.v
Figure 7: Evidence session with the Scottish Government on 9 February 2016  Source: Scottish Parliament
Source: Scottish Parliament
Professor Leese, who provided support to the Minister during the evidence session, also commented on this issue “…anecdotes often raise good clinical questions, which is exactly why we listen to anecdotes. Those questions then need to be tested, as I have said, in a clinical trial, to ensure that a therapy will have benefits”.v
Conclusions and recommendations
In the Committee's view, better means of capturing patient experience need to be developed so that clinicians can deliver the Scottish Government's aim to promote ‘realistic medicine’ that ensures the most appropriate treatment is given to patients. In this regard, the Committee recommends that the Scottish Government should develop guidance for listening exercises to ensure that they are designed in an impactful way and obtain best value for money.
This includes ensuring that the views of the relevant target group can be extrapolated from the results, for example, ensuring that Scottish patients’ views can be clearly identified from the results where participants that are not Scottish-domiciled take part. Listening exercises should also be designed to capture ‘anecdotal’ evidence in a way that can meaningfully inform the development of future clinical studies, clinical guidance or public policy (whichever is relevant to its aims). It is also recommended that the Scottish Government ensures that the participants receive feedback on the outcome of the listening exercise, including any specific action points that will be taken forward.
Overall conclusion
Thyroid conditions can have a significant impact on the lives of those who are diagnosed with having them. While the majority of patients with hypothyroidism will be diagnosed and treated successfully using the standard treatment and testing regimes, there is a proportion of patients for whom this is not the case.
This petition has raised the profile of that cohort of patients who either do not respond to the standard treatment, or who do not respond to the extent that they feel sufficiently well. It is important that these patients are believed when they report ongoing symptoms to clinicians.
However, the Committee recognises that there are differing views as to the evidential basis of both the current system of diagnosis, testing and treatment and the changes that are called for in the petition. The Committee recognises that discussions about these differing views will, and should, continue. This report sets out the Committee's conclusions and, where appropriate, makes recommendations for consideration by the Scottish Government and other decision makers. The Committee looks forward to receiving responses from these bodies.